Figure 8.
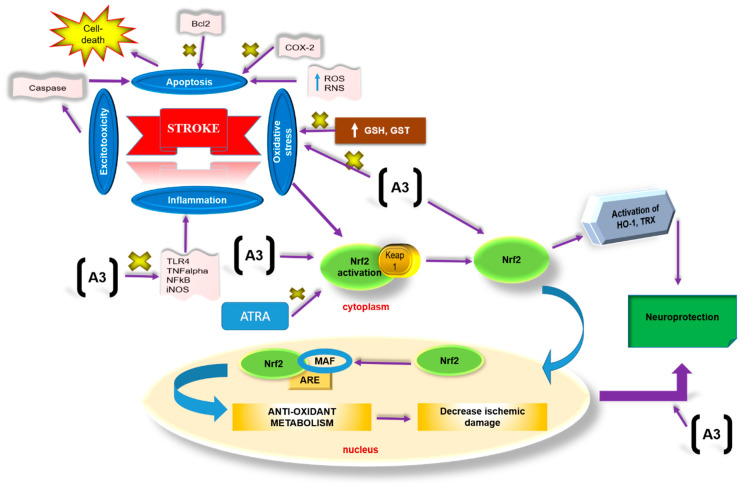
The graphical representation to elaborate on the possible mechanism underlying antioxidative and anti-inflammatory potential of A3 against the p-MCAO-induced brain injury. A3 causes Nrf2 activation and suppresses oxidative damage as indicated by elevated GST and GSH and reduced NO eventually leading to neuroprotection. ATRA blocks Nrf2 activation thereby leading to inhibition of endogenous antioxidative capacity. The majority of ROS and RNS are the products of oxygen metabolism in mitochondria. The superoxide anion produced is exported to the cytosol, and in the attempt to reduce the toxicity, some more reactive species are generated. These reactive species then cause degradation of DNA, lipids, and proteins. Under such conditions, the antioxidant response system (ARS) becomes activated to balance redox status. In our body, Nrf2 acts as a master regulator and is activated in response to stress via two mechanisms; a kinase-independent mechanism where reactive specie directly oxidize or nitrosylate the keap 1, a cysteine-rich protein that is bound to Nrf2 and thus releasing Nrf2. The other mechanism is kinase-dependent degradation, where a stressor such as glutamate, through activation of the Gq pathway, phosphorylate Nrf2. As a result, Nrf2 is transported into the nucleus where it displaces Bach1, a transcriptional repressor of ARE (antioxidant response elements), and heterodimerize with Maf and bind to ARE on DNA, eventually triggering transcriptional factors for NQO1, HO-1, and GST, thus increasing cellular defense against redox-modulators.