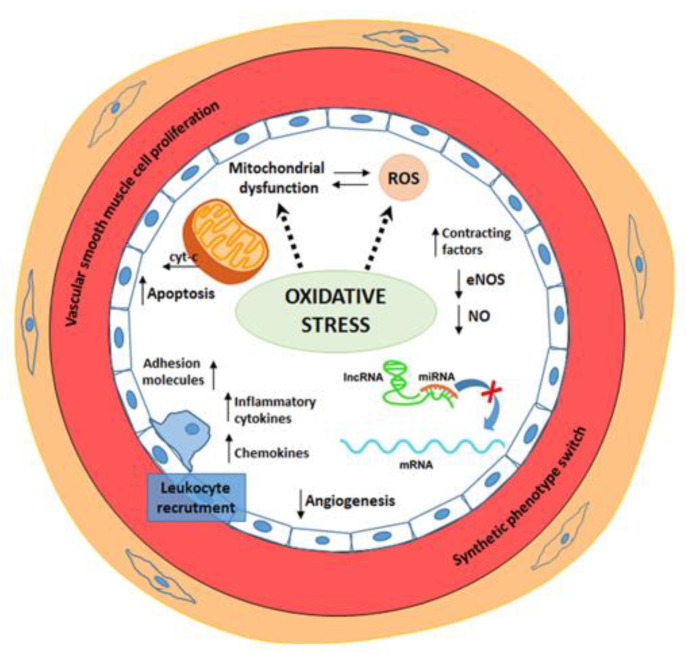
Figure 1.
Schematic representation of the main pathogenetic mechanisms involved in endothelial dysfunction. Endothelial dysfunction is defined as an impaired vasodilation and a proinflammatory and prothrombotic status. Endothelial dysfunction-induced phenotypic changes include eNOS inactivation and diminished availability of NO, up-regulated expression of adhesion molecules and increased chemokine secretion, leukocyte adherence, cell permeability, low-density lipoprotein oxidation, platelet activation, and vascular smooth muscle cell proliferation and migration. Inflammation-induced oxidative stress results in an increased accumulation of reactive oxygen species (ROS), mainly derived from mitochondria. Excessive ROS production causes oxidation of macromolecules inducing cell apoptosis mediated by cytochrome-c release (cyt-c). Oxidative stress increases vascular permeability, promotes leukocyte adhesion and induces alterations in endothelial signal transduction and redox-regulated transcription factors. Recently, alterations of gene regulatory mechanisms and deregulation of non-coding RNAs (miRNAs, lncRNA) have been reported to contribute to endothelial dysfunction.