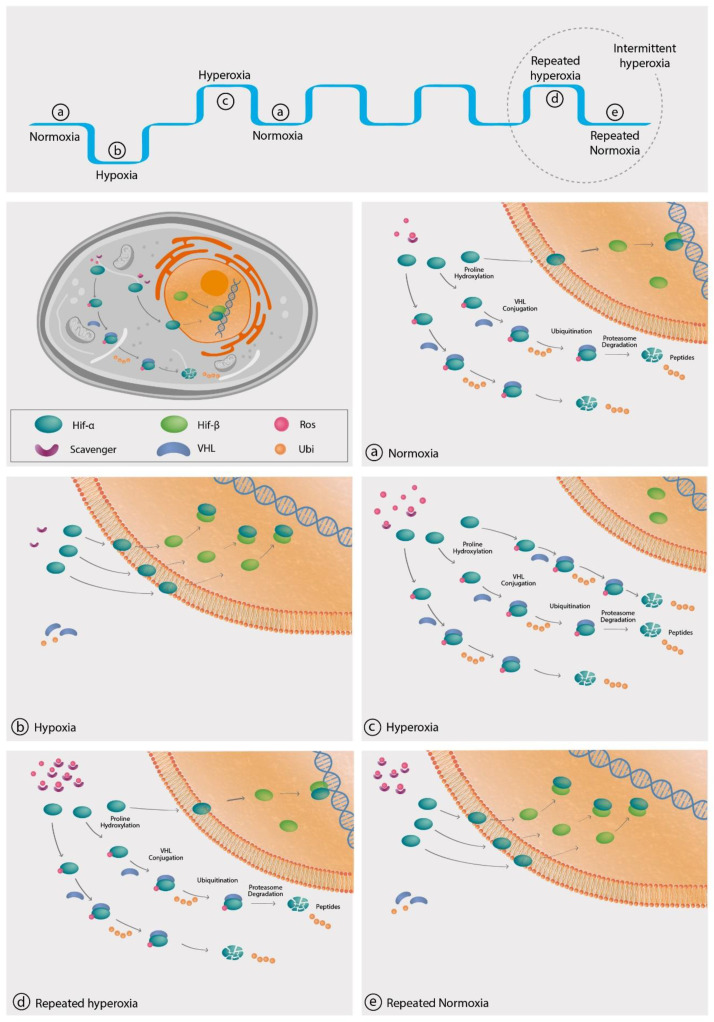
Figure 2.
The intracellular cascade of HIF-1 alpha. Legend: HIF-1 is a heterodimer composed of cytoplasmatic HIF-1α and the nuclear HIF-1β subunits. (a) Under normal oxygen environments, the ratio of ROS/scavenger is high and the free ROS molecules initiate HIF-1α hydroxylation, HIF-1α subunits become a target for VHLp (von Hippel–Lindau protein) protein which facilitates HIF-1α subunits ubiquitination and degradation. (b) Under hypoxic conditions, less oxygen and ROS molecules are available, HIF-1α subunits are not hydrolyzed, and more HIF-1α subunits penetrate the nucleus to conjugate with HIF-1β subunits and generate the active HIF transcription factor. (c) At the hyperoxic environment, more ROS and oxygen are available; thus more HIF-1α subunits are hydrolyzed and degraded. (d) The adaptive response to repeated hyperoxia includes increases in the production of scavengers that adjust to the increased ROS generation. Thus, the ROS/scavenger ratio gradually becomes similar to the ratio under normal oxygen environment prior to initiating repeated hyperoxic exposures. (e) Upon return to normoxia, following repeated hyperoxic exposures, the ratio of ROS/scavenger is low due to the fact scavengers elimination half-life (T1/2) is significantly longer than the T1/2 of ROS. Accordingly, less HIF-1α subunits are hydroxylated, and more of them penetrate the nucleus, conjugate with HIF-1β to generate the active HIF, similar to the hypoxic state.