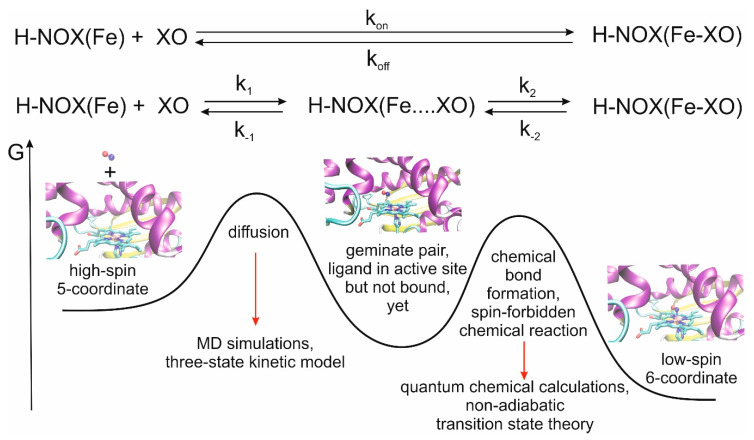
Figure 1.
Ligand binding by Heme-Nitric oxide and OXygen (H-NOX) proteins and definition of the relevant rate constants. Experimentally, and usually, kon and koff are determined, while calculations allow us to study the steps individually and obtain the values of k1, k−1, k2 and k−2. The overall process can be divided into two major events: diffusion of the ligand to the active site and the formation of the bond between the ligand and the Fe(II) ion. Due to the nature of the two events, different methodologies are required for their proper description, and as the spins are inverted on iron in the course of the chemical reaction, a special form of transition state theory is needed for the derivation of the rate constant values [15,22].