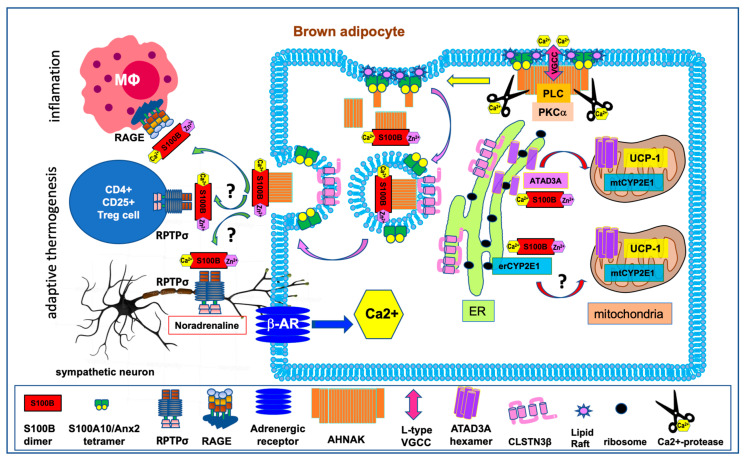
Figure 3.
Schematic representation of beta-adrenergic receptors signaling and S100B functions for mitochondrial biogenesis and adipokinesis in brown adipocyte. Mammalian BAT is made up of specialized adipocytes that express uncoupling protein 1 (UCP1), which dissipates the mitochondrial proton gradient, forcing increased flux through the electron transport chain and subsequent heat generation. Brown fat is innervated by nerve endings of the sympathetic nervous system. Sympathetic neurons release noradrenaline molecules that bind to and activate the β3-adrenergic receptors (β-AR) on fat cells. This activation triggers a cascade of biochemical events, such as changes in cytoplasmic Ca2+ concentration, elevations of intracellular cAMP levels, activation of kinases cascades, and mitochondrial biogenesis [56]. β-AR stimulation also induces S100B transcription [15]. The cytoplasmic Ca2+/Zn2+-bound S100B assists the synthesis, folding, stability and/or subcellular addressing of nuclear (p53) or mitochondrial (ATAD3A, CYP2E1) S100B binding partners involved in brown adipocyte differentiation and mitochondrial biogenesis. Changes in cytoplasmic Ca2+ concentration also induce AHNAK proteolysis and Ca2+-dependent exocytosis of S100B-containing vesicles by means of enlargosomes. In the extracellular space, it is hypothesized that S100B binds to RPTPσ receptors on sympathetic neurons and Treg cells to support the development of functional beige fat. S100B can also bind to RAGE on macrophages. The S100B–RAGE axis contributes to the cross-talk between adipocytes and immune cells and may play a role in inflammation associated with obesity. See also Section 5 for details).