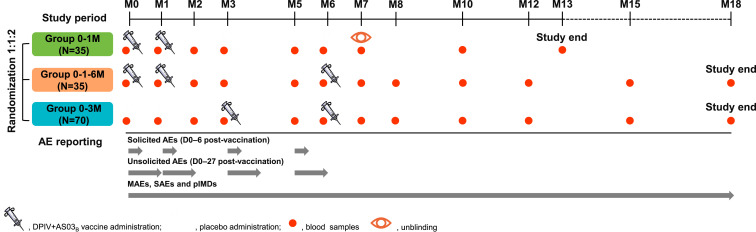
Figure 1.
Study design. AEs = adverse events; D = day(s); DPIV+AS03B = adjuvant system 03B-adjuvanted inactivated tetravalent dengue virus vaccine; M = month; MAEs = medically attended AEs; N = number of participants in each group included in the total vaccinated cohort; pIMDs = potential immune-mediated diseases; SAEs = serious AEs; 0–1 M = participants receiving two doses of DPIV+AS03B administered 1 M apart, at M0 and M1; 0–3 M = participants receiving two doses of DPIV+AS03B administered 3 M apart, at M3 and M6; 0–1–6 M = participants receiving three doses of DPIV+AS03B with the first two given 1 M apart and the third given 6 M after the first, at M0, M1, and M6. Only immunogenicity blood samples for analyses described here are shown in this figure.