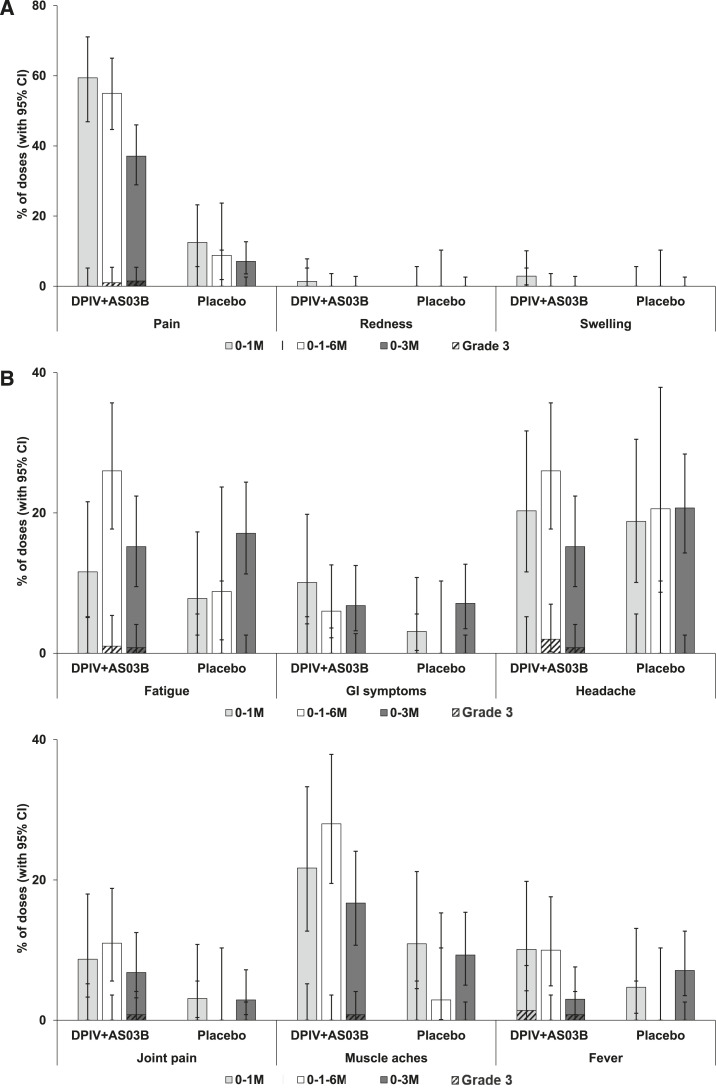
Figure 3.
Overall per dose incidence of solicited injection-site (A) and general (B) adverse events during the 7-D post-vaccination period (TVC). DPIV+AS03B = adjuvant system 03B-adjuvanted inactivated tetravalent dengue virus vaccine; GI symtoms = gastrointestinal symptoms (nausea, vomiting, diarrhea, and/or abdominal pain); M = month; TVC = total vaccinated cohort; 0–1 M = participants receiving two doses of DPIV+AS03B administered 1 M apart, at M0 and M1; 0–3 M = participants receiving two doses of DPIV+AS03B administered 3 M apart, at M3 and M6; 0–1–6 M = participants receiving three doses of DPIV+AS03B with the first two given 1 M apart and the third given 6 M after the first, at M0, M1, and M6. Error bars indicate 95% CIs.