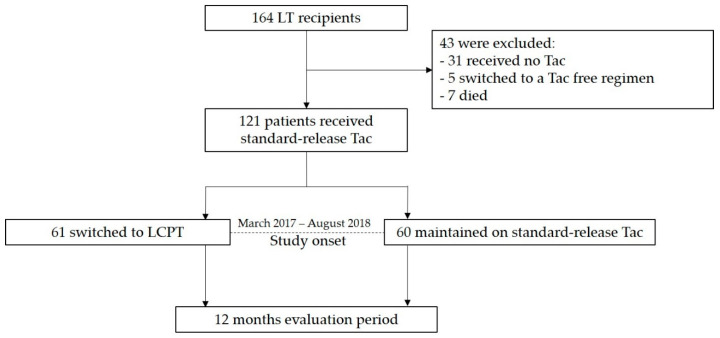
Figure 1.
Study design and patient enrolment. A total of 164 liver transplant (LT) recipients were screened for eligibility. Only LT recipients who were started on IR- or ER-Tac (standard-release tacrolimus) and continued taking this drug until the beginning of the study were included. During the enrolment period (March 2017–August 2018), 121 patients met the inclusion criteria and were either switched to LCPT (once-daily MeltDose® tacrolimus (Tac); intervention group) or maintained on standard-release tacrolimus (control group). Clinical data were analysed in a 12-month follow-up. We hypothesized that conversion from standard-release Tac to LCPT increases concentration/dose (C/D) ratio and thereby preserves renal function