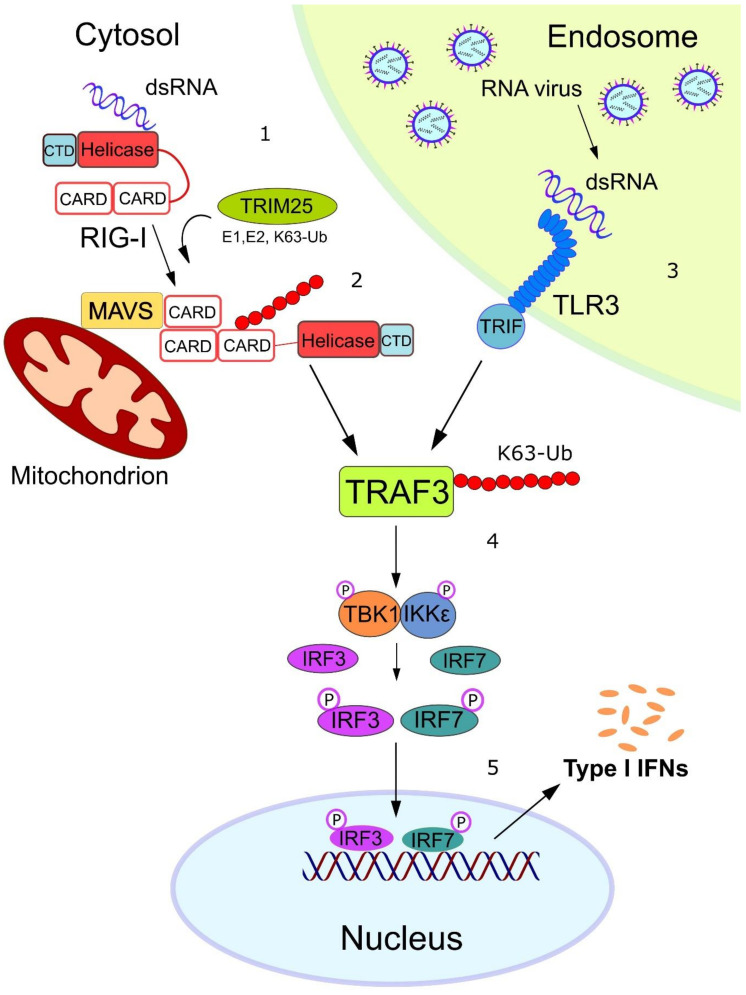
Figure 2.
An RNA virus activates the type I Interferon response. 1. The cytosolic RNA sensor RIG-I undergoes conformational changes after binding to short double-stranded (ds)RNA through its helicase domain and C-terminal domain (CTD). This event permits the N-terminal caspase activation and recruitment domain (CARD) of RIG-I to be exposed for TRIM25-mediated K63-linked ubiquitylation. 2. Ubiquitylated RIG-I can now bind to the CARD domain of mitochondrial antiviral signaling (MAVS) protein, which in turn leads to the recruitment of the E3 ligase TNF receptor-associated factor (TRAF)3 and its subsequent K63 ubiquitylation. 3. In parallel, long dsRNA can be sensed by the Toll-like receptor (TLR)3 receptor within the endosome, leading to the TRIF-mediated recruitment of the E3 ligase TRAF3, which in turn K63-autoubiquitylates itself. 4. K63-ubiquitylated TRAF3 acts as a scaffold protein for the recruitment and activation of the kinase complex TBK1/IKKε. 5. Activated TBK1/IKKε phosphorylates the transcription factors IRF3/IRF7, which translocate into the nucleus where they induce the expression of type-I interferon genes.