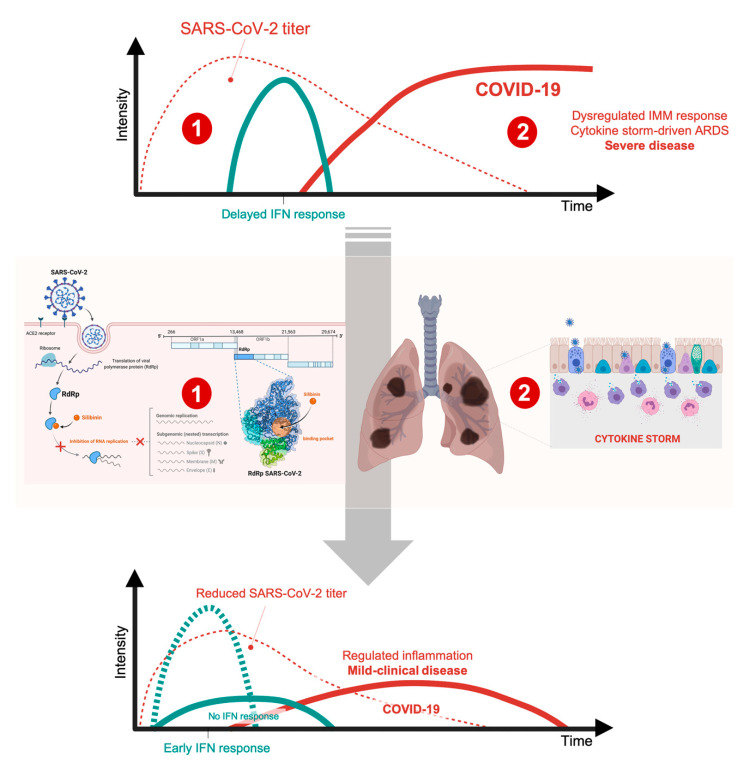
Figure 2.
Silibinin: a putative regulator of the too-little-too-late interferon response in COVID-19. Whereas it is well known that type I-interferon (IFN)-induced anti-viral response is among the earliest and most potent of the innate responses to fight viral infection, the timing of IFN-I response relative to virus replication might be key for SARS-CoV-2 infection outcome. (1) The extremely rapid and robust replication of SARS-CoV2—while IFN-I expression is delayed—is one of the initial triggers of lung inflammation, therefore highlighting the relevance of reducing initial viral load through direct anti-viral interventions. By targeting the central component of the replication/transcription machinery of SARS-CoV-2, namely the viral polymerase RdRp, silibinin is expected to reduce viral load and/or impede delayed interferon responses. The early antagonism of several SARS-CoV-2 proteins to the IFN response delays or prevents the innate immune response. Delayed IFN signaling, however, further orchestrates inflammatory monocyte/macrophage (IMM) responses and sensitize T-cells to apoptosis, which results in a further dysregulated inflammatory response, cytokine-driven acute respiratory distress syndrome (ARDS), and severe/fatal disease in a subgroup of COVID-19 patients. (2) Silibinin might be expected to phenotypically integrate the mechanisms of action of IL-6-targeted monoclonal antibodies and pan-JAK1/2 inhibitors to alleviate the pathological accumulation of inflammatory macrophages as the source of the cytokine storm and T-cell lymphopenia associated with fatal COVID-19 disease. We acknowledge that the proposed viral RdRp-targeted (1) and host STAT3-targeted (2) mechanisms of silibinin are highly intertwined. On the one hand, the (pro-/anti-) responses of STAT3 signaling to virus infection are complex both in a virus-specific and in a stage-specific manner in the virus lifecycle. On the other hand, the host IFN response could promote the ability of SARS-CoV-2 to maintain cellular targets in neighboring human upper airway epithelial cells via up-regulation of the SARS-CoV-2 receptor ACE2 in a STAT1-related manner. As the competition phenomena and distinct dynamics of the STAT3/STAT1 duo in pro- (e.g., IFN-I) and anti-inflammatory pathways might constitute an evolutionary-conserved mechanism of the host to tightly control the immune response while avoiding tissue damage, further studies should clarify whether STAT3 participates in the net protective or detrimental role of type I IFN depending on the stage of SARS-CoV-2 infection. In animal models, IFN-I administration shortly after SARS-CoV infection has positive effects and protects mice from lethal infection, whereas later administration IFN-I fails to inhibit viral replication and has side-effects (i.e., severe COVID-19-like increased infiltration and activation of monocytes/macrophages/neutrophils in the lungs accompanied by enhanced pro-inflammatory cytokine expression) that result in fatal pneumonia from an otherwise sublethal infection. Accordingly, although promising findings have been observed upon IFN-I treatment or IFN-based combination therapy with lopinavir/ritonavir, ribavirin, or remdesivir in pre-clinical animal models, mixed results have been found in humans. Understanding if STAT3 is one of the host restriction/promoter factors targeting SARS-CoV-2 lifecycle in a IFN-I/ACE2-dependent/independent manner may provide better strategies to dissociate the dual roles of IFN-I in SARS-CoV-2 infection.