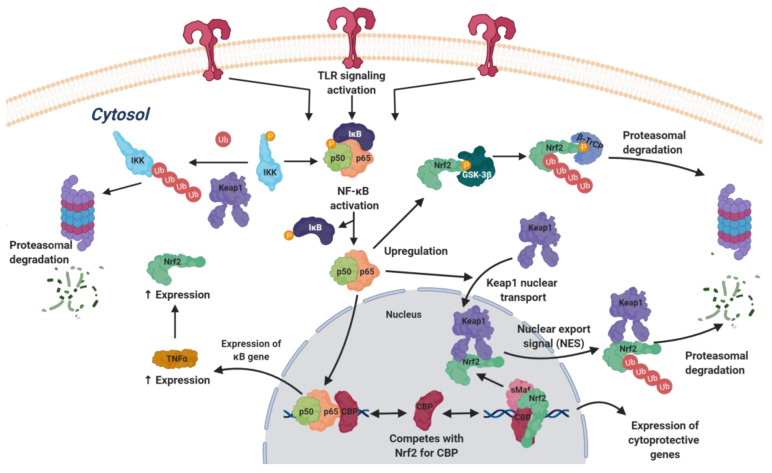
Figure 5.
Crosstalk between the nuclear factor-κB (NF-κB) and NRF2 pathways. Upon TLR activation, NF-κB is liberated from its natural repressor, IκB, by phosphorylation promoted by the IKK (IκB kinase) complex. Liberated NF-κB dimer rapidly translocates into the nucleus where it binds to CREB binding protein (CBP), competing with NRF2, and it initiates the expression of proinflammatory genes including tumor necrosis factor-α (TNF-α) citokine. NF-κB drives a negative regulation over NRF2 through its monomer p65 by different pathways. p65 promotes the nuclear importation of KEAP1, where it binds to NRF2, concluding the expression of phase II-related genes. Then, the KEAP1–NRF2 complex is exported to the cytosol and coupled to the Cul3 ubiquitin ligase that induces the proteasomal degradation of NRF2. Additionally, GSK-3β directly phosphorylates NRF2, coding its degradation though interaction with β-TrCP. β-TrCP is also able to induce the degradation of IκB (inhibitor of nuclear factor kappa B), increasing the activity of NF-κB and thus limiting NRF2 activation. On the other hand, KEAP1 stabilizes IκB by inducing the degradation of IKK via proteasomal degradation. Thus, it helps to close the crosstalk between NF-κB and NRF2, creating a fine-tuned loop between inflammation and the antioxidant response.