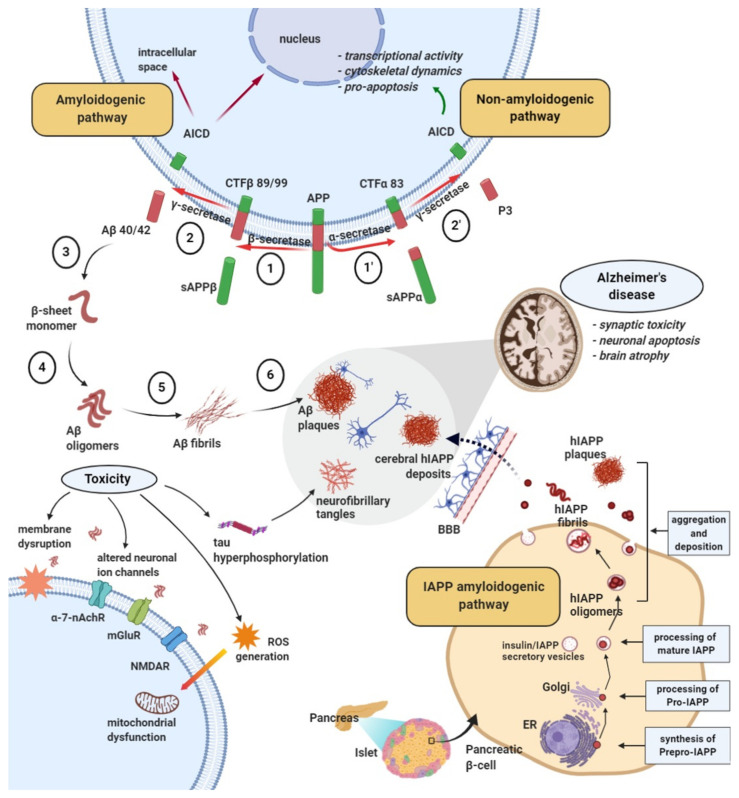
Figure 3.
Amyloidogenic pathways of amyloid-beta peptide development in Alzheimer’s disease and islet amyloid polypeptide in diabetes. (1) Proteolytic cleavage of the transmembrane glycoprotein β-amyloid precursor protein (APP) by beta-secretase (amyloidogenic pathway) into the extracellular sAPPβ and the membrane β-C-terminal membrane-bound (CTF-β) 89/99 or (1′) by alpha secretase resulting into extracellular sAPPα and membrane CTF-α 83 fragment (non-amyloidogenic pathway). (2) Further cleavage of the CTF-β fragments at multiple sites by γ-secretase, resulting in the formation of extracellular Aβ 40–42 monomers and intracellular APP intracellular domain (AICD) that can translocate to the nucleus (2′) CTF-α cleavage by γ-secretase generating non-toxic extracellular P3 peptide and intracellular AICD. (3) Aβ conformational rearrangement from α-helical structure to β-sheet secondary structure monomers. (4) Assemblage of β-sheet Aβ monomers into soluble oligomers responsible for neuronal toxicity through mitochondrial dysfunction and membrane receptor binding. (5) Gradual aggregation of Aβ oligomers into insoluble amyloid fibrils and (6) deposits of amyloid senile plaques characteristic of AD. The initially 89 amino acid long islet amyloid polypeptide (IAPP) pre-pro peptides/along with the pro-insulin precursor/are synthesized in the endoplasmic reticulum of the pancreatic islet β-cells and then processed by enzymatic cleavage at both C-terminal and N-terminal to 67 amino acid pro-IAPP peptide. The pro-IAPP intermediate is subsequently cleaved by enzymes/prohormone convertase in the Golgi apparatus and in the secretory granules to form the 37 amino acid IAPP molecules. Mature IAPP stored together with insulin in the secretory granules may undergo misfolding processes, leading to potentially toxic intracellular and subsequent extracellular IAPP oligomers. Overexpression of the human IAPP molecules further induces IAPP aggregation into amyloid fibrils and plaques deposited intra- and extracellularly. IAPP oligomers may enter in the brain by crossing the blood–brain barrier, and IAPP deposits may contribute to AD pathology. Aβ, amyloid-β peptide; APP, amyloid precursor protein; AICD, cytoplasmic polypeptide; BACE1, beta-secretase 1; BBB, blood–brain barrier.