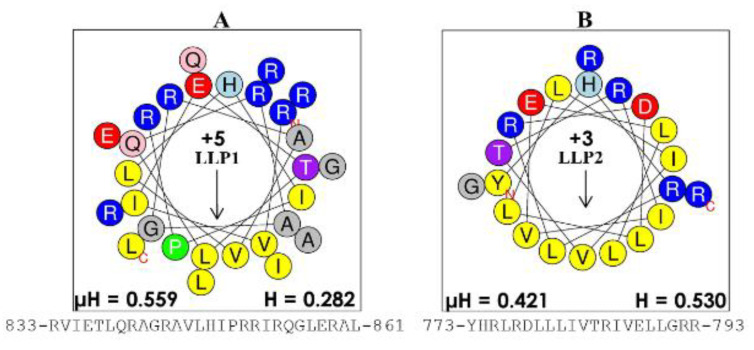
Figure 2.
Structures of lentivirus lytic peptides (LLPs). The intracellular domains of the gp41 protein were identified as potential antimicrobial peptides because of their characteristic cationic amphipathic motif, with the helical wheel of (A) the more potent antibacterial LLP-1 (charge of +5) and (B) that of LLP-2 (charge of +3). These LLP domains set the foundation for the engineered AMPs, which we termed engineered cationic antimicrobial peptides (eCAPs) (Figure 3). The number on the left and the number on the right of each sequence indicate the beginning and the end of the primary sequence, respectively, in the protein gp41; µH, hydrophobic moment; H, hydrophobicity derived from the online software heliquest.fr.