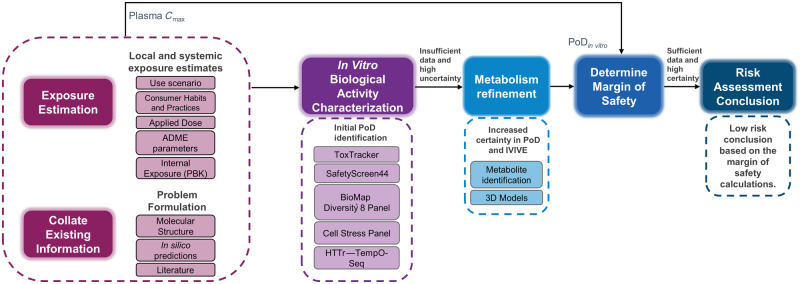
Figure 1.
Next-Generation Risk Assessment case study workflow for 0.1% coumarin in consumer products. Initial steps involved collating existing data, generating in silico predictions, and problem formulation. In parallel, applied and systemic consumer exposure estimates were calculated based on the use scenario, habits and practices information, and chemical parameters. A battery of in vitro assays was then conducted to characterize the cellular response to coumarin. From these data, point of departure (PoD) values with associated uncertainties were determined, however, the lack of metabolic capacity of the cell line models used, and the potential toxicity of reactive metabolites led to the generation of additional data (metabolism refinement). All PoDs were compared with exposure estimates (plasma Cmax) to calculate a margin of safety, which was used for the risk assessment decision. Abbreviations: HTTr, high-throughput transcriptomics; IVIVE, in vitro to in vivo extrapolation.