Figure 1.
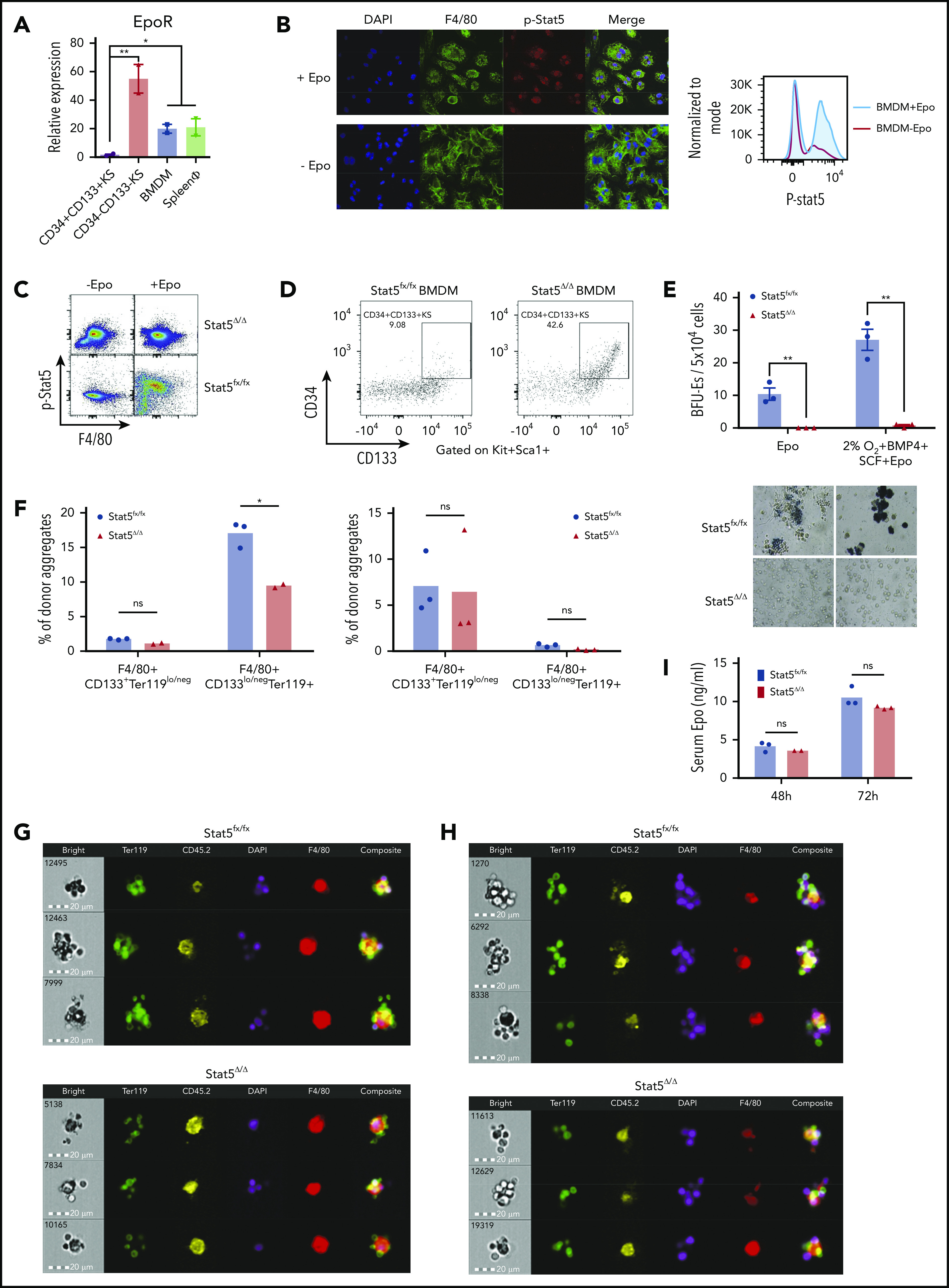
Differentiation of SEPs requires activation of macrophages through Epo-dependent Stat5 activation. (A) mRNA expression of EpoR in sorted SEPs (CD34+CD133+KS and CD34–CD133–KS), BMDMs, and sorted primary F4/80+ spleen macrophages (spleen ϕ). (B) Immunocytochemistry analysis of colocalization of phosphorylated Stat5, F4/80, and 4′,6-diamidino-2-phenylindole (DAPI) in BMDMs (left). Flow cytometry analysis of phosphorylated Stat5 in BMDMs with or without Epo (right). (C) Flow cytometry analysis of Stat5 phosphorylation in F4/80+ spleen macrophages isolated from Stat5fx/fx or Stat5Δ/Δ mice with or without Epo. (D) Flow cytometry analysis of CD34+CD133+KS SEPs cocultured with Stat5fx/fx BMDMs or Stat5Δ/Δ BMDMs. CD34 and CD133 expression is shown on cells gated on Kit+Sca1+. (E) Stress BFU-E colony number (top) and representative BFU-E morphology (bottom) of SEPs cocultured with Stat5fx/fx BMDMs or Stat5Δ/Δ BMDMs. (F) Quantification of flow cytometry analysis of early-stage F4/80+CD133+Ter119lo/neg EBIs and late-stage F4/80+CD133lo/negTer119+ EBIs after 48 hours of phenylhydrazine (PHZ) treatment (left) and 72 hours of PHZ treatment (right). F4/80, CD133, and Ter119 expressions are shown on aggregates gated on CD45.2+ (donor). (G-H) Representative FlowSight analysis of EBIs after (G) 48 hours of PHZ treatment and (H) 72 hours of PHZ treatment (n = 2-3 mice per group). (I) Serum Epo level measurement by enzyme-linked immunosorbent assay (ELISA) after PHZ treatment. Student t test (2-tailed). Data represent means ± standard error of the mean (SEM). ns, not significant. *P < .05; **P < .01.
