Figure 3.
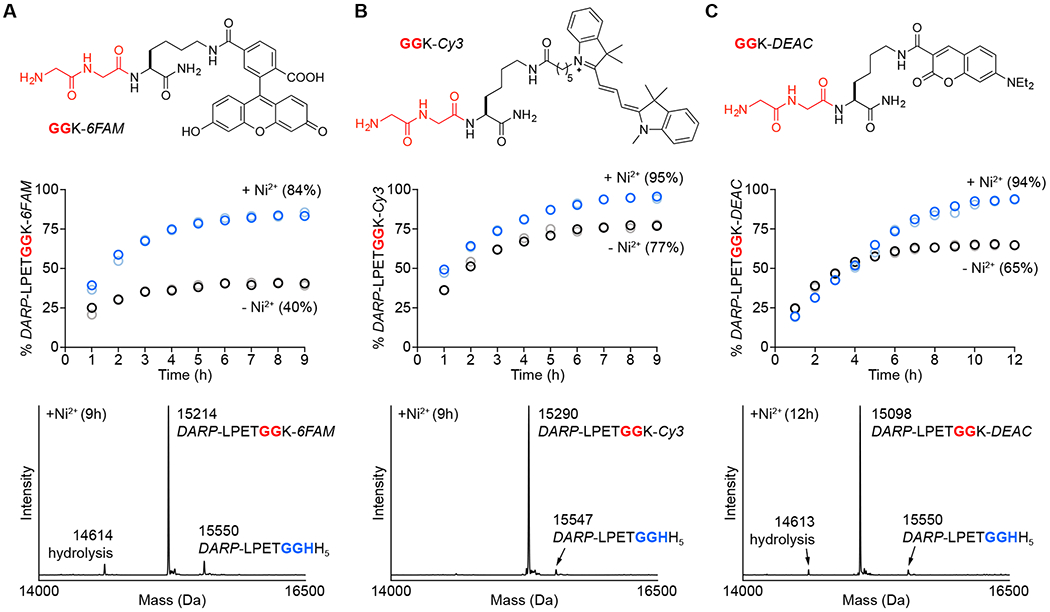
Comparison of MA-SML versus standard sortase-mediated ligation for fluorescent labeling of a DARPin substrate (DARP-LPETGGHH5). Diglycine nucleophiles (top) modified with (A) fluorescein (GGK-6FAM), (B) cyanine 3 (GGK-Cy3), or (C) 7-(diethylamino)coumarin (GGK-DEAC) were reacted with DARP-LPETGGHH5 in a 1:1 molar ratio using 20 mol% SrtAstaph in the presence or absence of 4 equivalents of NiSO4. The addition of Ni2+ significantly improves product formation (middle) as determined by LC-ESI-MS (blue/light blue circles represent two independent reactions containing Ni2+, black/grey circles represent two reactions in the absence of Ni2+; values in parentheses represent average % product formation for the two independent trials at the final timepoint). Deconvolved mass spectra (bottom) of the 9 or 12 h timepoints for reactions with Ni2+ confirm formation of the desired conjugates, with minimal contamination from unreacted DARP-LPETGGHH5 and competing substrate hydrolysis (calculated MWs: DARP-LPETGGHH5 = 15550 Da, hydrolysis = 14613 Da, DARP-LPETGGK-6FAM = 15213 Da, DARP-LPETGGK-Cy3 = 15294 Da, DARP-LPETGGK-DEAC = 15098 Da).
