Figure 4.
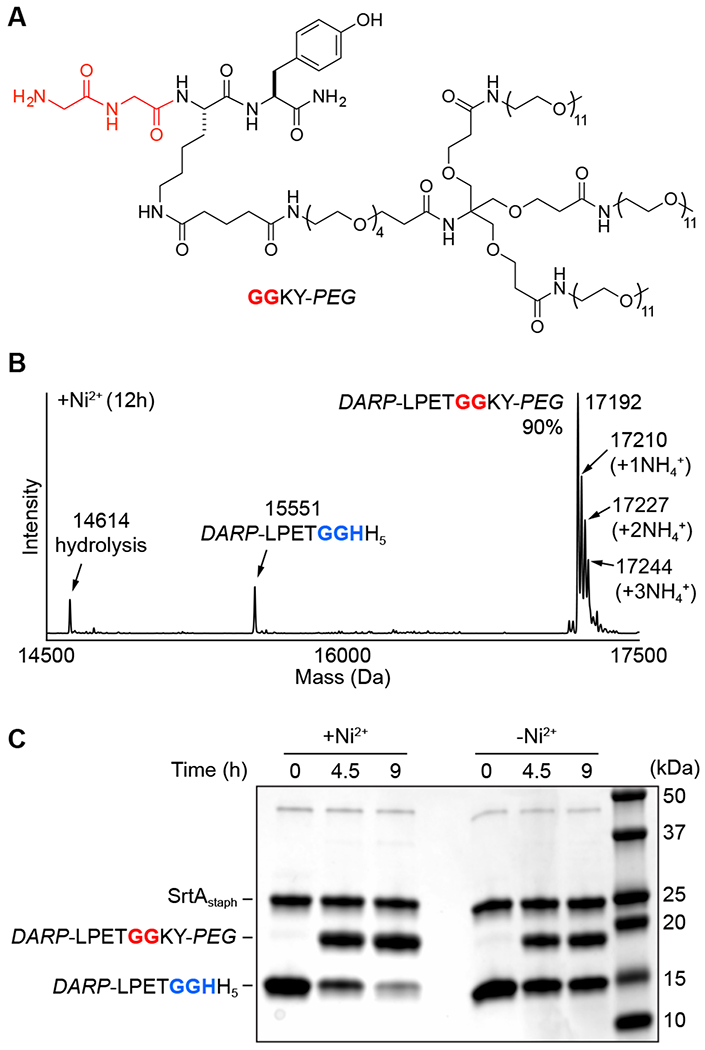
(A) Structure of PEG-modified diglycine nucleophile (GGKY-PEG) for attachment of a branched PEG unit via MA-SML. (B) Representative deconvoluted mass spectrum of 12 h timepoint from MA-SML reaction utilizing 20 mol% SrtAstaph, 4 equivalents of NiSO4, and a 1:1 stoichiometry of DARP-LPETGGHH5 and GGKY-PEG. The extent of product formation (90%) was estimated from peak areas derived from the deconvoluted mass spectrum. Peak areas for the observed ammonium adducts were included as part of the total product formed (calculated MWs: DARP-LPETGGHH5 = 15550 Da, hydrolysis = 14613 Da, DARP-LPETGGKY-PEG = 17191 Da). (C) SDS-PAGE gel demonstrating higher PEGylation in the presence of Ni2+ for reactions using a 1:1 ratio of DARP-LPETGGHH5 to GGKY-PEG.
