Figure 5.
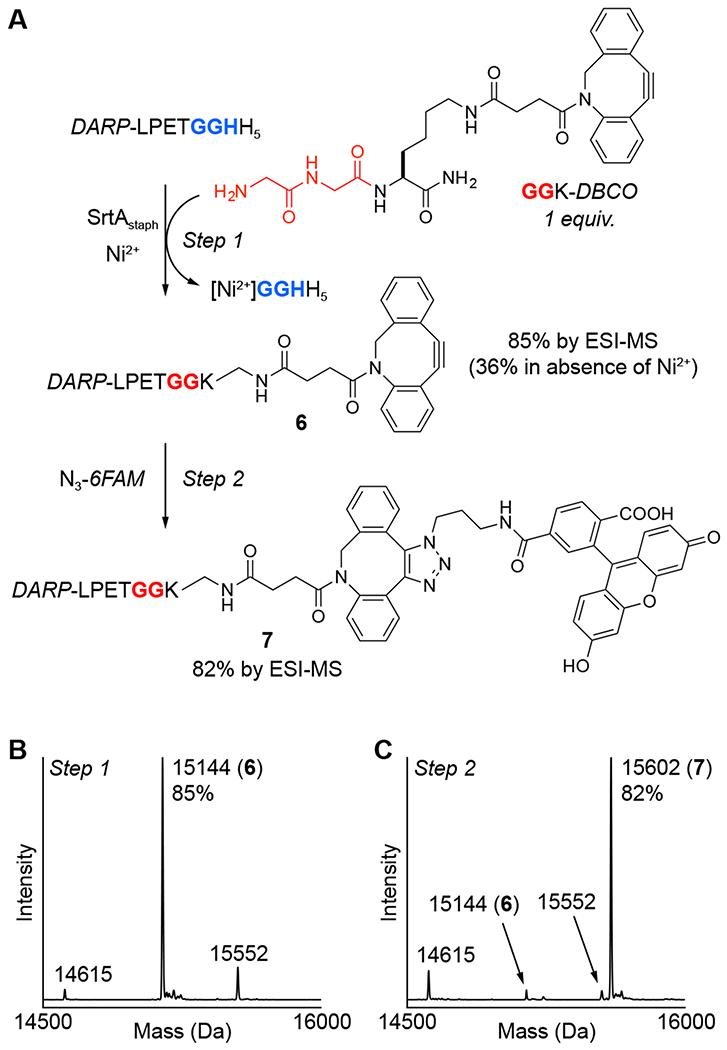
One-pot strategy for sequential MA-SML and strain-promoted azide-alkyne cycloaddition (SPAAC). (A) An initial MA-SML reaction using 20 mol% SrtAstaph, 4 equivalents of NiSO4, and a 1:1 ratio of DARP-LPETGGHH5 and GGK-DBCO generates the desired DBCO conjugate (6) with more than a two-fold increase in reaction conversion compared to controls lacking Ni2+. Direct addition of two equivalents of fluorescent azide (N3-6FAM) to the crude MA-SML reaction mixture produces the final SPAAC product (7) as 82% of the total crude protein mixture. Deconvoluted mass spectra of the crude reaction mixtures from the (B) MA-SML (spectrum shown is for the 9 h reaction time point) and (C) SPAAC steps confirm formation of the desired protein conjugates (calculated MWs: DARP-LPETGGHH5 = 15550 Da, hydrolysis = 14613 Da, DARP-LPETGGK-DBCO (6) = 15142, SPAAC product (7) = 15600 Da).
