Figure 6.
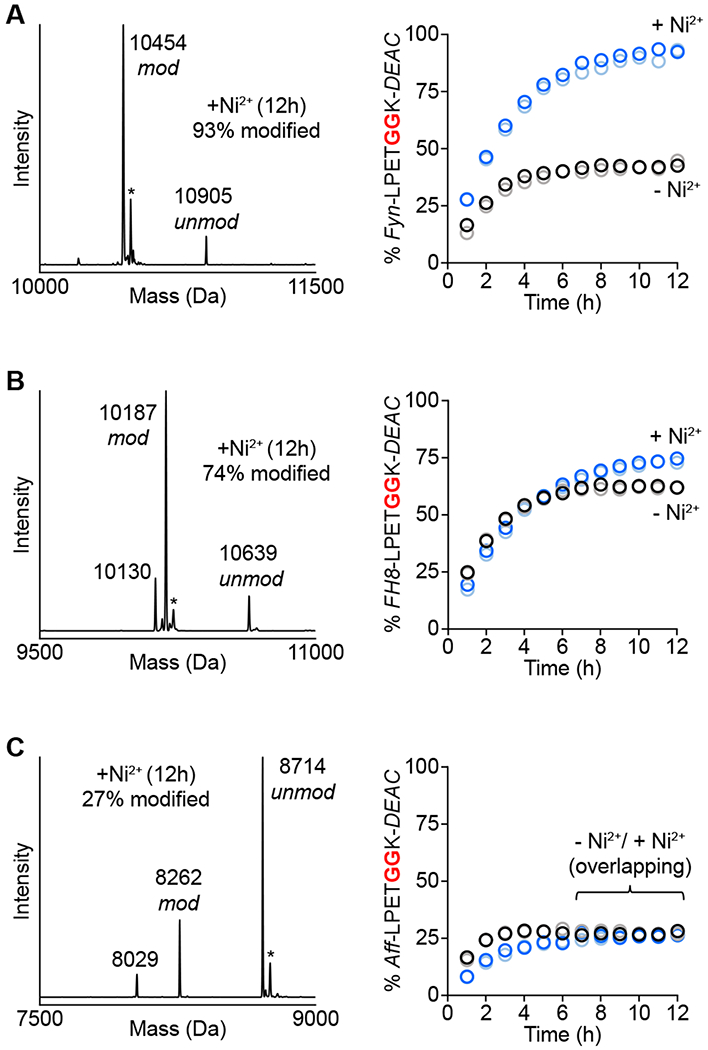
MA-SML versus standard sortase-mediated ligation for modification of additional protein targets. One equivalent of coumarin nucleophile (GGK-DEAC) was reacted with (A) Fynomer (Fyn-LPETGGHH5), (B) FH8 (FH8-LPETGGHH5), or (C) Affibody (Aff-LPETGGHH5) using 20 mol% SrtAstaph in the presence or absence of Ni2+. The addition of Ni2+ improves reaction conversion (right) for Fynomer and FH8 substrates as estimated by LC-ESI-MS (blue/light blue circles represent two independent reactions containing Ni2+, black/grey circles represent two reactions in the absence of Ni2+). Representative deconvoluted mass spectra (left) of MA-SML reactions at 12 h confirm formation of the desired DEAC conjugates (calculated MWs: modified/unmodified Fyn-LPETGGHH5 = 10454/10907 Da, modified/unmodified FH8-LPETGGHH5 = 10187/10640 Da, modified/unmodified Aff-LPETGGHH5 = 8261/8714 Da). Additional notes: * = MeCN adducts from LC-ESI-MS mobile phase; peak at 10130 Da in B is consistent with the FH8-LPETGGK-DEAC conjugate lacking a glycine residue (−57 Da); peak at 8029 Da in C corresponds to a truncated Affibody substrate that co-purified with full length Aff-LPETGGHH5.
