Figure 2.
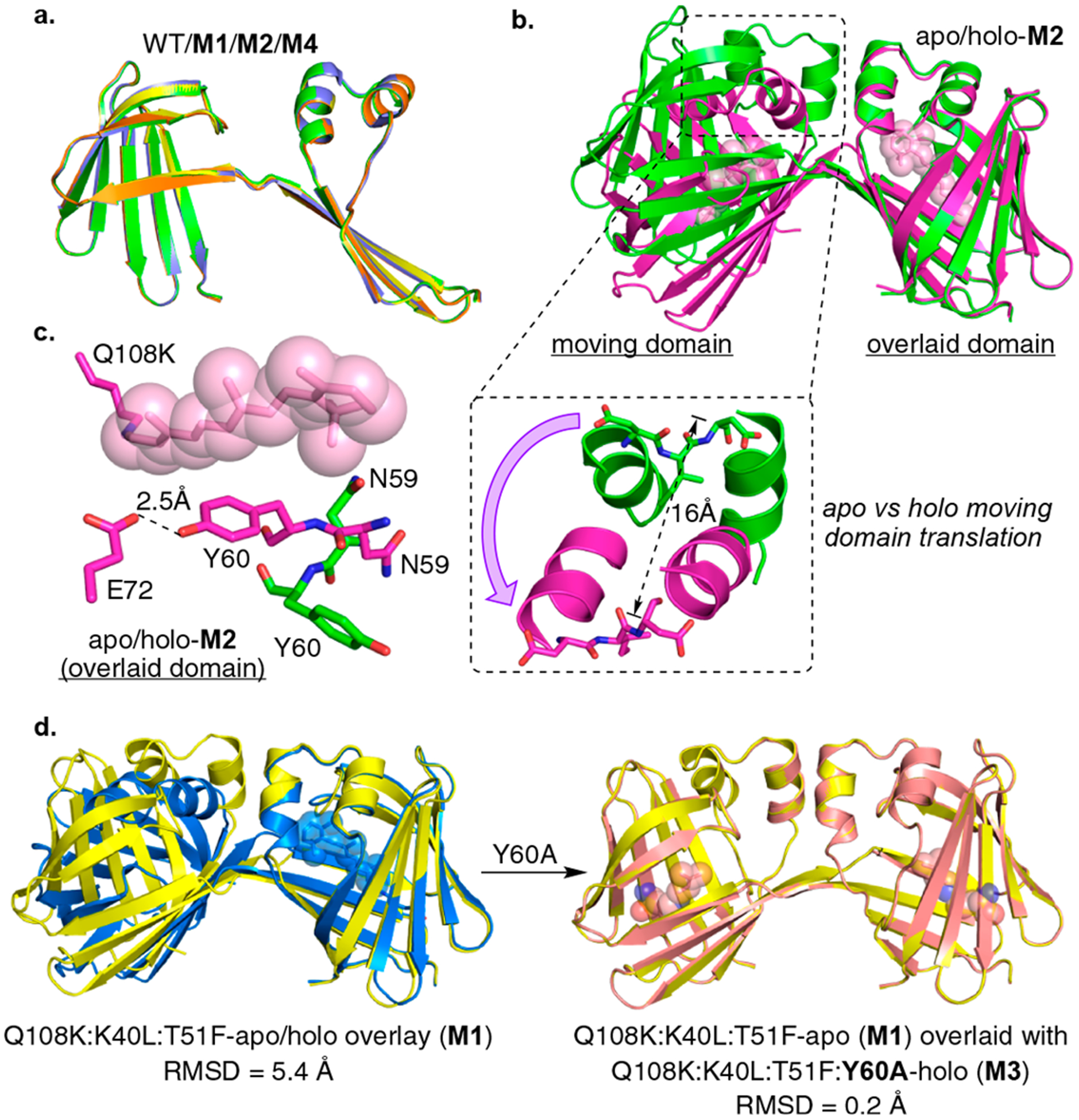
(a) Overlay of the single chains of three apo dimers (con-A): WT (blue), Q108K:K40L:T51F (M1, yellow), Q108K:T51D (M2, green), and Q108K:K40L:T51W (M4, orange) illustrating excellent overlap of all. (b) Overlay of apo- and holo-M2 structures (con-A green, and con-B purple, respectively) illustrating the large change in the relative orientations of the two domains upon ligand binding (here, as in all dimer overlays, only one of the two domains of the dimer is overlaid, allowing the change in relative orientation of domains to be visualized). As shown in the expansion, this results in a 16 Å movement of the α-helix. Retinal (pink) is shown in space filling representation. (c) The binding pocket of apo-M2 (con-A, green C atoms) and holo-M2 (con-B, magenta C atoms, retinal is shown in space filling representation (pink)). Allosteric conformational change is driven by the orientation of the Tyr60 and Asn59 side chains. A flipped-in Asn59 would sterically clash with the bound retinylidene, leading to the flipped-out conformation of Asn59 and flipped-in conformation of Tyr60. (d) Left: An overlay of apo-M1 (con-A, yellow) and holo-M1 (blue, con-B) showing a large conformational change in the relative orientation of two dimer domains similar to the one shown above for the apo vs holo M2. Right: An overlay of apo-M1 (con-A, yellow) and holo-M3 (magenta, con-A) showing no change in the relative orientation of the two domains upon ligand binding.
