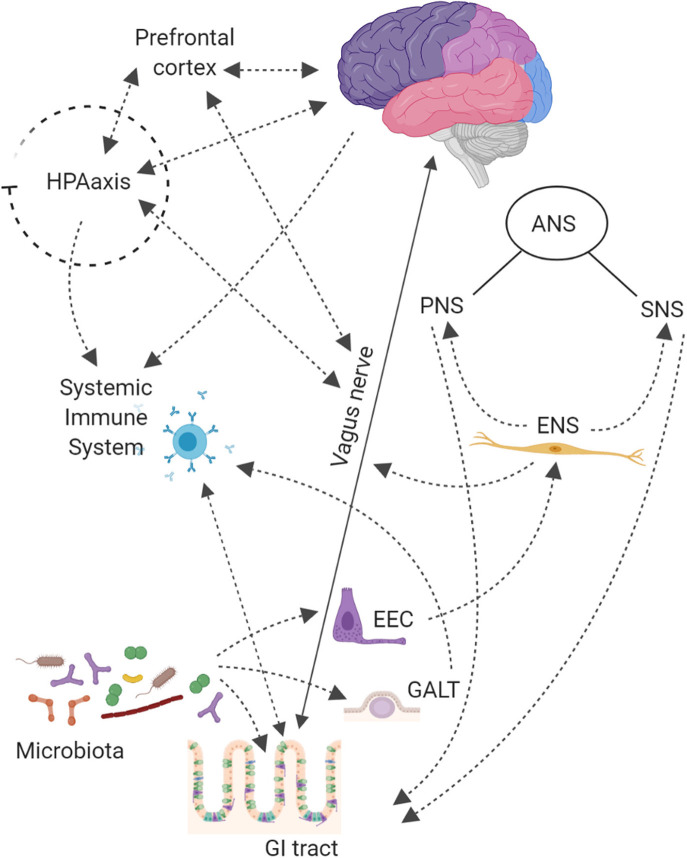
Figure 1.
The Central Nervous System (CNS) is connected with the gut thought Autonomic Nervous System (ANS), that is divided into: Sympathetic (SNS), Parasympathetic (PNS), and Enteric (ENS) Nervous Systems. The SNS exerts mainly an inhibitory effect on GI tract and regulates GI blood flow. The PNS exerts both excitatory and inhibitory control over gastric and intestinal tone and motility. The main component of PNS is the Vagal Nerve that affects the peristalsis and the sphincter muscles of gut, and by afferent spinal and vagal sensory neurons. It sends feedbacks from the GI tract to the brain stem, which in turn engages the hypothalamus and limbic system for data processing. Finally, the ENS, located in the walls of the GI tract and described as a “second brain” (an extensive system composed of about 500 million neurons, exceeding those at the level of the spinal cord), communicates with the CNS via SNS and PNS. The ENS displays sophisticated coordination and exhibits plasticity and learning in response to changing dietary habits. In the intestine up to 80,000 vagal afferent nerve terminations are present, which send and receive signals, to and from the brain in a rate of 9:1. The Hypothalamic-Pituitary-Adrenal (HPA) axis coordinates adaptive responses to stress and activate memory and emotional centers in the limbic system of the brain and is also able to influence the composition of the gut microbiota and increase GI permeability. In the case of pathogen invasion, tissue damage, or homeostatic dysregulation, signals are processed by the CNS and sent to sympathetic and efferent vagal nerves, that release catecholamine and acetylcholine, respectively, under control of immune cells. The HPA axis through release of corticosteroids, with anti-inflammatory and immunosuppressive effects, regulates brain–immune pathway. The Gut-Associated Lymphoid Tissue (GALT) comprises 70% of the body's immune system; chronic stress has been shown to alter intestinal permeability (i.e., leaky gut syndrome), which is associated with a low-grade inflammation that can be functionally linked to several neurological disorders. On the other hand, also gut microbiota contributes to connect the gut to the brain compartment. Bacterial products can stimulate enteroendocrine cells (EEC) to produce several neuropeptides that can enter the bloodstream and/or directly influence the ENS, activate immune cells that release cytokines or activate the vagal nerve. This can affect the physiology of the brain, including neurotransmission and neurogenesis, but may also be involved in neuroinflammation.