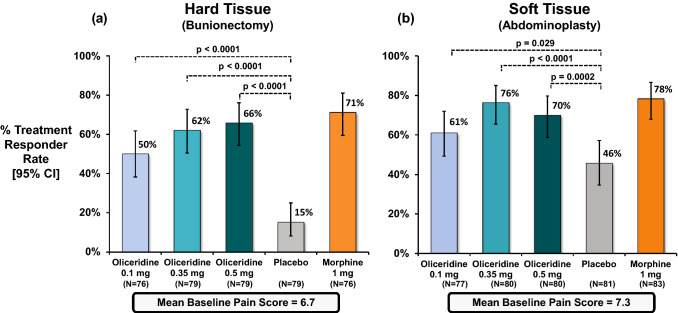
Fig. 1.
Primary treatment response from controlled phase III trials. The primary endpoint analysis compared the percentage of treatment responders in each oliceridine regimen with the percentage of responders in the placebo regimen. a Hard tissue (bunionectomy) study and b soft tissue (abdominoplasty) study. The duration in the hard tissue (bunionectomy) study was 48 h and in the soft tissue (abdominoplasty) study was 24 h. A patient was considered a treatment responder if all the following conditions were met: at least a 30% improvement in their final time-weighted sum of pain intensity difference (SPID) from baseline at 48 h (for bunionectomy) or 24 h (for abdominoplasty); without rescue pain medication during the randomized treatment period; without early discontinuation of study medication for any reason; without reaching the study medication dosing limit. *P vs. placebo (Hochberg adjusted), CI confidence interval