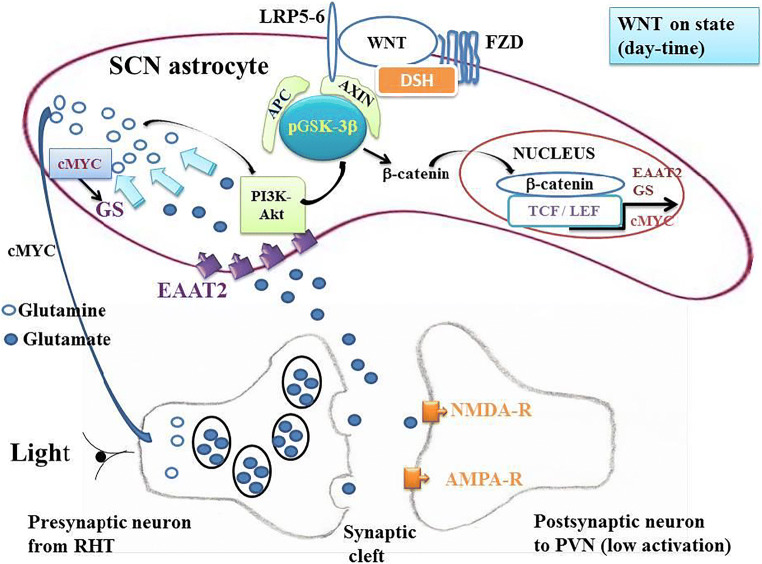
Fig. 2.
Phosphorylated glycogen synthase kinase-3β (pGSK-3β) and canonical WNT/β-catenin signaling in “on” state. The canonical WNT pathway is “on-state” in the presence of the inactive phosphorylated form of the glycogen synthase kinase-3β (pGSK-3β). Phosphorylation of GSK-3β can be induced either by WNT ligands or by other molecular mechanisms and particularly by light. On the one hand, a WNT ligand binds both FZD and LRP5/6 receptors. GSK-3β is then under the inactive phosphorylated form pGSK-3β. This leads to activation of the phosphoprotein Disheveled (DSH). DSH recruits the destruction complex (pGSK-3β + AXIN +APC) to the plasma membrane, where AXIN directly binds the cytoplasmic tail of LRP5/6. APC is the adenomatous polyposis coli. On the other hand, expression of pGSK-3β follows a circadian rhythm and reaches a maximum in the morning at ZT2. In both mechanisms, β-catenin is detached from the destruction complex, accumulates into the cytosol, and then translocates to the nucleus to bind the LEF-TCF co-transcription factors. This induces the WNT-response gene transcription of numerous genes such as EAAT2, GS, and cMYC. This favors the re-uptake of glutamate from the synaptic cleft to the cytoplasm of astrocytes. This leads to a decrease of glutamate content in the synaptic cleft and a decrease of the activation of NMDA-R and AMPA-R, which are receptors located in the synaptic part of the glutamatergic post-synaptic neuron. SCN neurons project to the NTS via PVN and consequently decrease the parasympathetic tone and increase the sympathetic tone which favors the increase in BP in the morning when the expression of pGSK-3β is increased. β-Catenin increases the expression of cMYC. cMYC stimulates GS. Glutamine in the SCM astrocyte cytoplasm stimulates the PI3K-Akt pathway. The PI3K-Akt pathway phosphorylates GSK-3β