Fig. 1.
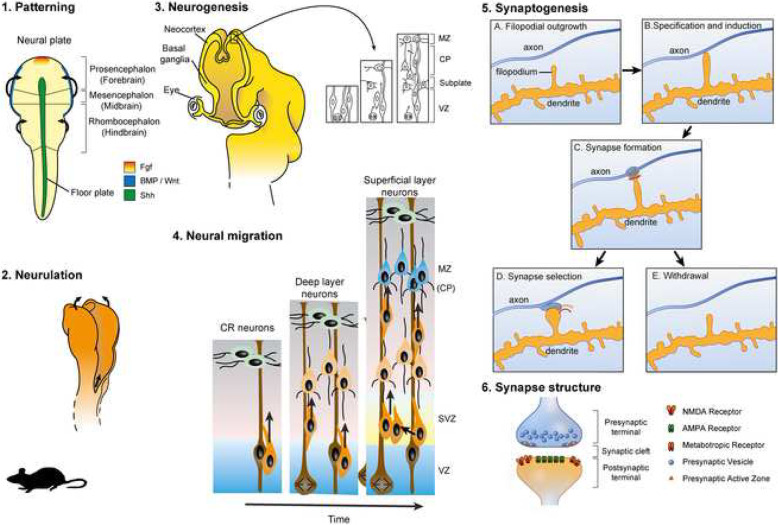
Embryonic development of the cerebral cortex: a primer. The development of the cerebral cortex can be divided into several stages (see figure). 1. Patterning: The basic plan of the mammalian brain is laid out at the neural plate stage. At this stage, signaling centers that surround the neural plate produce signaling molecules including FGFs, BMPs, and SHH which form a set of intersecting gradients across the neuroectoderm. Combinations of these signals are believed to confer specific regional fate on neural plate cells [61]. Next, in the process of neurulation (2), the edges of the neural plate fold towards each other and fuse together, thus forming the neural tube. At the earliest stages, the neural tube contains three primary brain vesicles, the prosencephalon (forebrain), mesencephalon (midbrain), and the rhombencephalon (hindbrain) [71]. The prosencephalon expands disproportionately, becoming larger than the other vesicles. Each vesicle subsequently gives rise to specific parts of the brain. For example, the prosencephalon will give rise to the cerebral cortex, ventral telencephalon, thalamus, and hypothalamus [152]. In the early neural tube, neuroepithelial progenitor cells divide symmetrically at the ventricular edge, giving rise to two daughter progenitors. These proliferative divisions rapidly expand the pool of neural progenitors [47, 122]. Neuroepithelial progenitor cells subsequently transform to form other progenitor types, primarily radial glia. Radial glia may divide either symmetrically or asymmetrically, giving rise to a radial glial and a neuronal daughter, thus initiating the process of neurogenesis (3) in which cortical neurons are born. As development proceeds, an increasing proportion of radial glia divide asymmetrically, generating large numbers of neurons. Another important population of neural progenitors, known as intermediate progenitors or apical progenitors, are found in the subventricular zone (SVZ) [47, 100, 101, 140]. Intermediate progenitors then continue to divide, making more neurons. Newborn neurons migrate (4) radially (indicated by black arrows) towards the outer (pial) edge of the embryonic cortex guided by a scaffold provided by radial glial fibers that project from the ventricular edge to the pial surface. Early-born neurons populate the deepest layers of the cortex. Later born neurons migrate past them, progressively building up the characteristic six-layered of the cortex, in the process of lamination [122, 123, 132, 140]. Neurons in each cortical layer have distinct molecular signatures, associated with their specific functions. Excitatory (glutamatergic) cortical neurons are generated in dorsal telencephalon, but inhibitory (GABAergic) cortical neurons are born in the ventral telencephalon, form where they migrate tangentially into the forming cortex. Once cortical neurons have migrated to their final destinations, they next form connections with their appropriate synaptic partners in the process of synaptogenesis (5), the first step in neural circuit assembly. During synaptogenesis, cell adhesion molecules such as neuroligins and neurexins are recruited to the site of the future synapse where they form a bridge between the axon and dendrite. This initiates protein specialization to organize the active zone of the presynaptic terminal and the post synaptic density (PSD) over a period of hours to days [106]. During this process, scaffolding proteins such as membrane-associated guanylate kinases (MAGUKs), PSD95, and SHANK1 are recruited to the site of axo-dendritic membrane contact [14, 69]. Next is the process of synapse stabilization (6). In rodents, thousands of synapses and dendritic spines per neuron are added in the period of 1-2 weeks of development but the majority of the synapses are removed or withdrawn and neuronal activity plays a key role in this [54, 109]. Many of the proteins located in the developing PSD play a role in synapse stabilization as many were shown to regulate synapse number and size. Neuronal connections could be between neurons from other brain structures that are further away (long-ranged connectivity) or with neurons from the same region of the brain (local connectivity). These connections are not final as many connections are made throughout embryonic and early development of the brain which will then be refined later on in development as connections that are used more are strengthened (activity-based neural connections) while connections that are less used are pruned as describe in the process of synapse stabilization to establish mature neural circuits [131, 142]. Figure is modified, with permission, from Price et al. (2017)