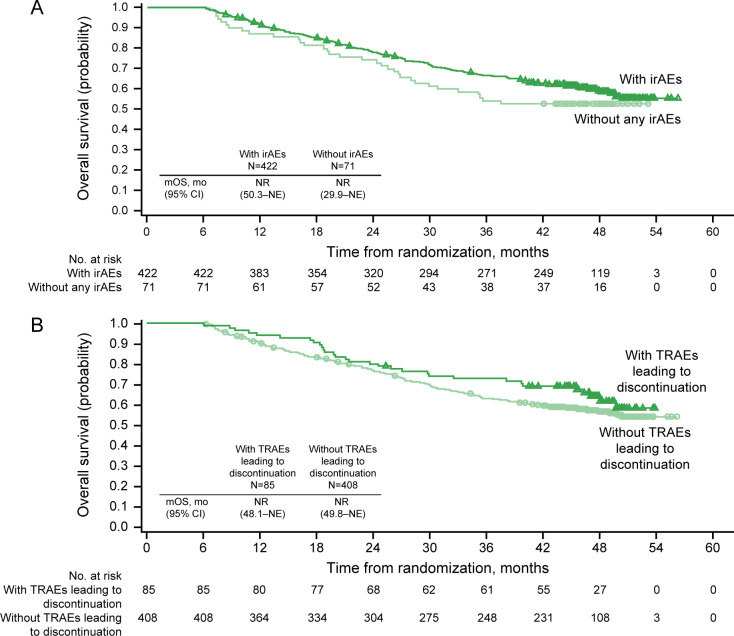
Figure 5.
Six-month landmark overall survival analyses in intent-to-treat patients with nivolumab plus ipilimumab. (A) Immune-related adverse events (irAEs), yes versus no. (B) Treatment-related adverse events (TRAEs) leading to discontinuation, yes versus no. Includes events reported between first dose and 30 days after last dose of study therapy. mOS, median overall survival; NE, not estimable; NR, not reached.