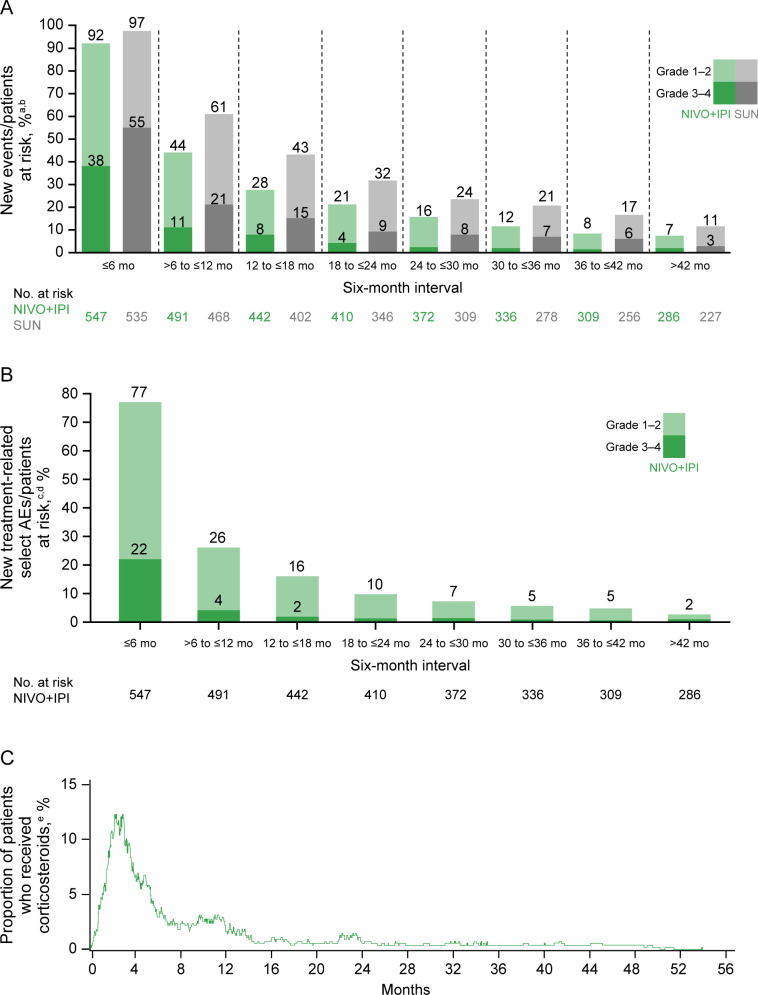
Figure 6.
Safety outcomes over time. (A) Treatment-related adverse events (AEs) over time by 6-month interval with nivolumab plus ipilimumab (NIVO+IPI) versus sunitinib (SUN). (B) Select treatment-related AEs over time by 6-month interval with NIVO+IPI. (C) Corticosteroid use over time with NIVO+IPI. aN=patients at risk at the beginning of each 6-month interval, and patients may be counted more than once across intervals. bIncidence of grade 3–4 treatment-related AEs in all intervals with NIVO+IPI after 24 months was ≤2.4%. cN=patients at risk at the beginning of each 6-month interval, and patients may be counted more than once across intervals. dIncidence of grade 3–4 treatment-related select AEs in all intervals with NIVO+IPI after 12 months was ≤1.6%. e≥40 mg prednisone daily or equivalent. Treatment-related AEs and treatment-related select AEs were calculated by 6-month interval using the total number of new events out of the total number of patients at risk at the beginning of the interval.