Figure 1. Lysosomal dysfunction and oxidized dopamine accumulation in PD patient iPSC-derived dopaminergic neurons.
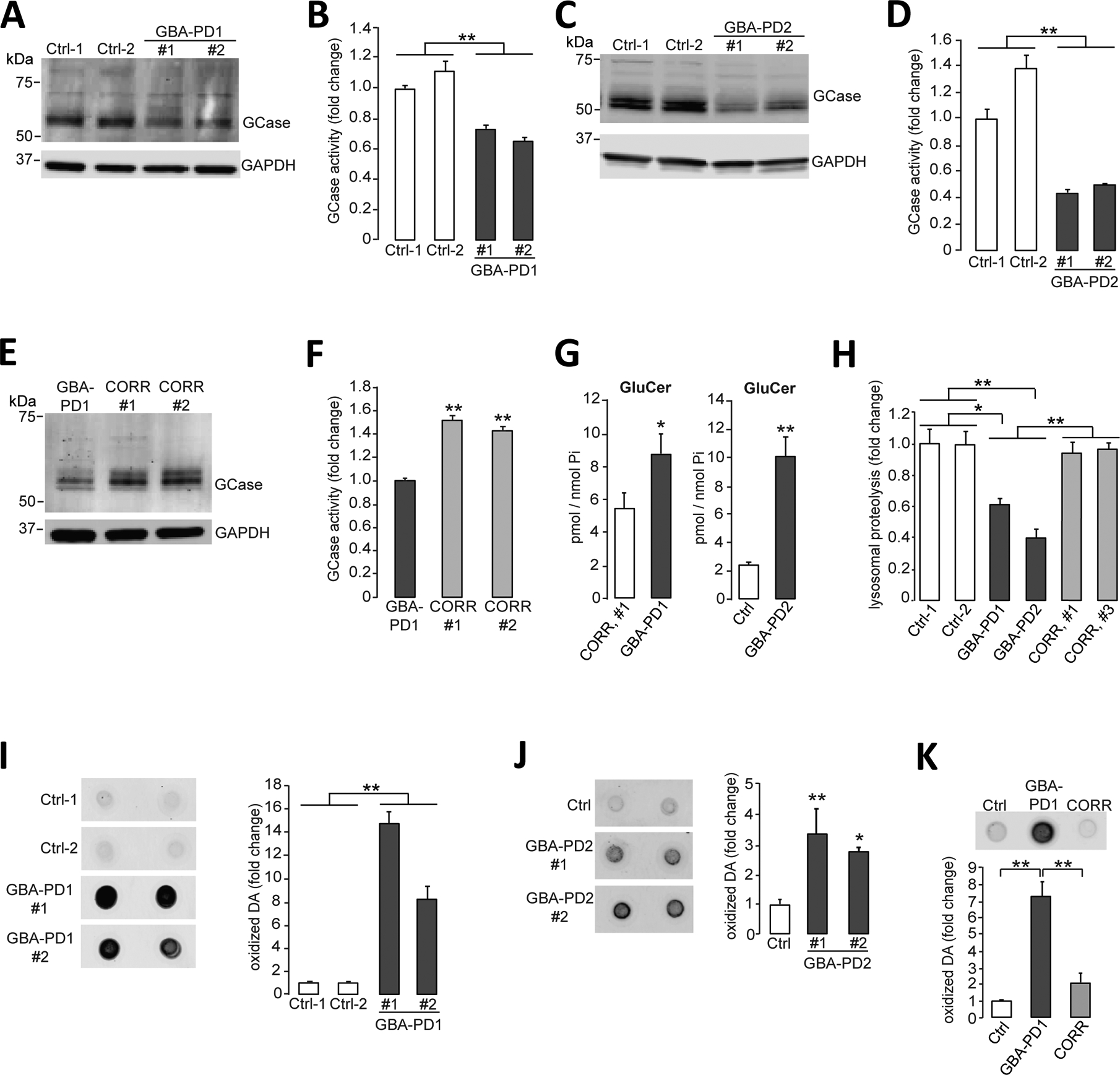
(A and B) Triton-soluble lysates of iPSC-derived dopaminergic neurons obtained from fibroblasts from healthy control individuals or patients with PD carrying the heterozygous 84GG GBA1 mutation (GBA-PD1, clone #1 and clone #2) were prepared at day 80 of differentiation. Neuronal lysates were analyzed for (A) GCase protein amounts by immunoblotting and (B) GCase activity by in vitro enzyme activity assay (N=3 independent experiments). (C and D) Triton-soluble lysates of iPSC-derived dopaminergic neurons obtained from fibroblasts from healthy control individuals or patients with PD carrying the heterozygous 84GG GBA1 mutation (GBA-PD2, clone #1 and #2) were prepared at day 60 of differentiation. Neuronal lysates were analyzed for (C) GCase protein amounts by immunoblotting and (D) GCase activity by in vitro enzyme activity assay (N=3 independent experiments). (E and F) Triton-soluble lysates from GBA-PD1 iPSC-derived dopaminergic neurons and isogenic control iPSC-derived dopaminergic neurons with the GBA1 mutation corrected by CRISPR-Cas9 gene editing (CORR, clone #1 and #2) were prepared at day 60 of differentiation. Neuronal lysates were analyzed for (E) GCase protein amounts by immunoblotting (representative blot shown) and (F) GCase activity by in vitro enzyme activity assay (N=3 independent experiments). (G) Quantification of intracellular glucosylceramide (GluCer) by mass spectrometry normalized to internal phosphate (Pi) was performed in GBA-PD1 mutant iPSC-derived dopaminergic neurons and CRISPR-Cas9-corrected GBA1 isogenic control iPSC-derived dopaminergic neurons (CORR, clone #1) (left, N=3 to 4 independent experiments). Quantification of intracellular glucosylceramide (GluCer) by mass spectrometry normalized to internal phosphate (Pi) was performed in GBA-PD2 mutant and healthy control iPSC-derived dopaminergic neurons (Ctrl) (right, N=3 independent experiments) at day 70 of differentiation. (H) Quantification of lysosomal proteolysis of long-lived proteins was measured by radioactive pulse-chase at day 80 of differentiation in healthy control (Ctrl), in GBA-PD1 and GBA-PD2 iPSC-derived dopaminergic neurons, and in CRISPR-Cas9 gene-edited GBA1 isogenic control iPSC-derived dopaminergic neurons (CORR, clone #1 and #3) (N=4 independent experiments). (I to K) Detection and quantification of oxidized dopamine (DA) by near-infrared fluorescence assay was performed (I) in healthy control iPSC-derived dopaminergic neurons (Ctrl) and GBA-PD1 mutant iPSC-derived dopaminergic neurons (clone #1 and #2) at day 80 of differentiation (N=3 independent experiments), (J) in healthy control iPSC-derived dopaminergic neurons (Ctrl) and GBA-PD2 mutant iPSC-derived dopaminergic neurons (clone #1 and #2) at day 60 of differentiation (N=5 to 6 independent experiments), and (K) in healthy control (Ctrl) and GBA-PD1 mutant iPSC-derived dopaminergic neurons and CRISPR-Cas9 gene-edited GBA1 isogenic control iPSC-derived dopaminergic neurons (CORR) at day 50 to130 of differentiation (N=3 independent experiments). Error bars, mean ± SEM. *P<0.05 and **P<0.01, Student’s t test (G) or one-way ANOVA with Tukey post hoc test (B, D, F, and H to K).
