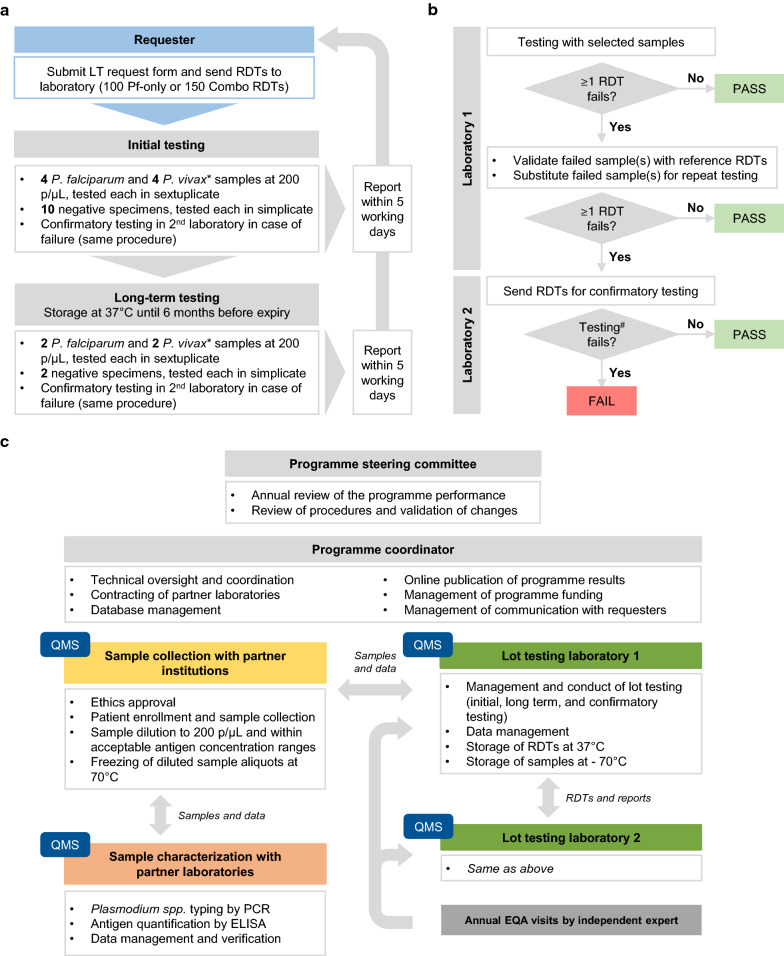
Fig. 1.
Overview of the Lot Testing process. a Schematic overview of the overall lot testing process, based on samples at 200 parasites per microlitre of blood (p/µL) and with antigen concentrations within a standardized range corresponding to this parasitaemia, *indicates a condition that applies to combination tests only. b Schematic overview of the testing procedure and pass/fail criteria. RDTs must detect all repeats of all samples at 200 p/µL in order to pass. False positives and anomalies, such as red background, flow failure, etc., are reported as comments and not used for pass/fail decisions. Insufficient buffer is reported as a “fail” result, #confirmatory testing in a second laboratory, if necessary, is performed according to the same two-step procedure than for the initial testing and using a different set of samples. c Main components and activities required for the LT programme are indicated. LT = Lot Testing, Pf = P. falciparum, EQA = External quality assessment, QMS = Quality management system