Figure 2.
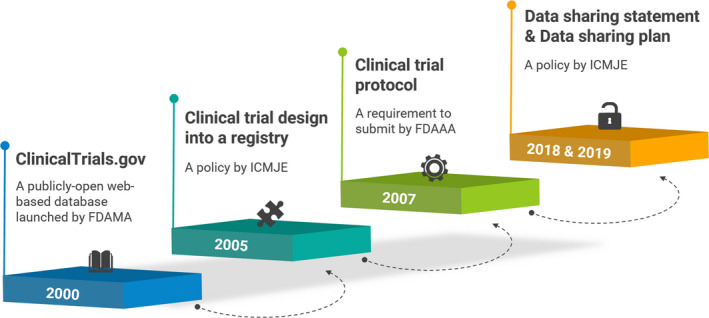
Accomplishments of establishing public systems and guidance for data sharing in clinical trials. FDAAA, Food and Drug Administration Amendments Act; FDAMA, Food and Drug Administration Modernization Act; ICMJE, International Committee of Medical Journal Editors.
