Figure 2.
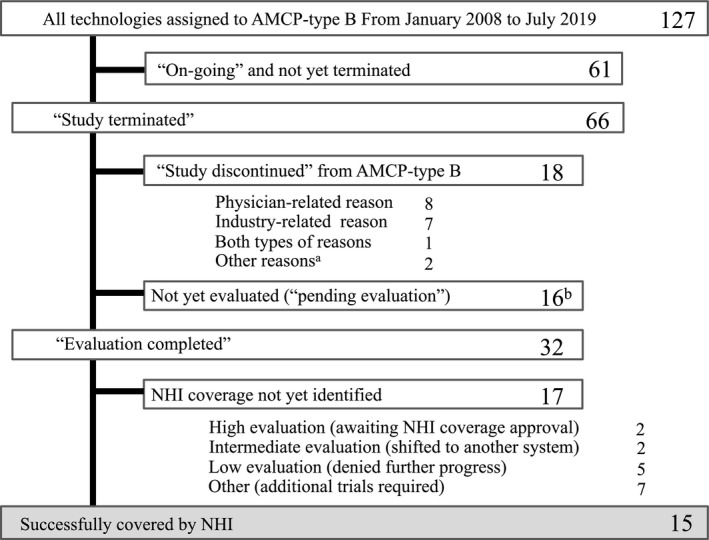
Flowchart for all technologies designated as Advanced Medical Care Program (AMCP)‐type B from January 2008 to March 2019. Of 66 studies that were conformed to terminate, evaluations were completed in 32. The technologies in 15 of these studies were introduced into National Health Insurance (NHI) coverage, whereas 17 were not. The details of the 15 covered technologies are shown in Table 1. Seventeen were not yet identified for NHI coverage, but two of these were evaluated as “high” and were awaiting pharmaceutical approval. Another two of these technologies, evaluated as “intermediate,” were confirmed and introduced through a different system (a Japanese system of companionate use: patients dispensation care (n = 1) or a special insurance framework (n = 1)). Five technologies that were evaluated as “low” were not promoted for regulatory approval or introduced into NHI coverage. Seven technologies were to undergo additional study, including investigator‐initiated clinical trials or another AMCP‐type B in the next phase (n = 7). One study was judged as inappropriate for fast‐track approval (n = 1), and 18 were discontinued for development via AMCP‐type B because of physician‐related reasons (n = 8), industry‐related reasons (n = 7), or both types of reasons (n = 1). Two technologies were introduced into NHI coverage by medical insurance revision before the technologies were evaluated through AMCP‐type B. aThese technologies were introduced into NHI coverage through medical fee revision in 2018 before completing AMCP‐type B clinical trials. bThis includes cases where the process of evaluation was unclear.
