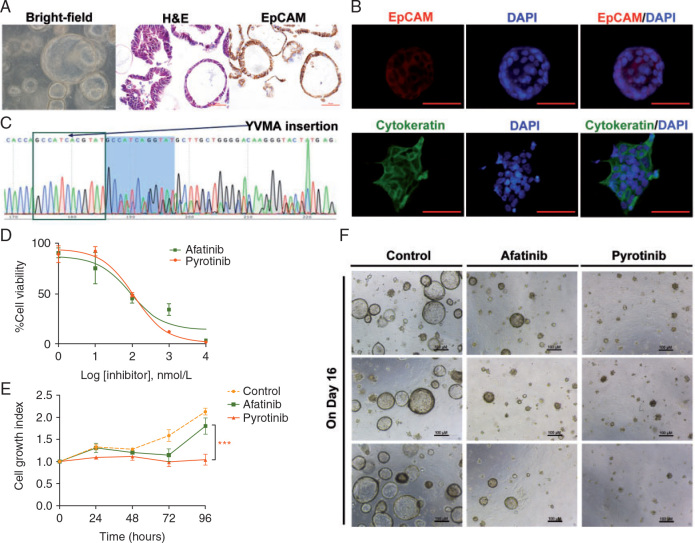
Figure 1.
Activity of pyrotinib in HER2-mutant patient-derived organoids. (A, B) 3D organoids in bright field (10 × 10), and H&E and IHC results of representative organoids suggested epithelial origin. (C) Sequencing results of representative organoids showed HER2 A775_G776insYVMA, indicating that the genomic characterization of this patient-derived in vitro models was identical to the parental tumor. (D) Drug response curves of the organoids treated with the dual EGFR/HER2 inhibitors (afatinib and pyrotinib, respectively) showed that the IC50 of afatinib and pyrotinib were 89.1 and 112.5 nM. Cell viability was measured by a CellTiter-Glo (Promega) Luminescent Cell Viability Assay after 72 h of treatment. (E) Cell growth curves of the organoids treated with vehicle (DMSO), afatinib (29 nM#) and pyrotinib (180 nM#) showed that compared with afatinib, pyrotinib at plasma concentration inhibited the cell growth more significantly (*P = 0.0038). #The concentrations were adopted from the in vivo plasma concentrations of the 2 drugs in previous phase I clinical studies, e.g. (Cafatinib: 21.1 ng/ml)/(MWafatinib: 718.08 g/mol)×1000 = 29 nM; (Cpyrotinib: 147 ng/ml)/(MWpyrotinib: 815.22 g/mol)×1000 = 180 nM. (F) Representative images (three repeated well) of the 3D organoids treated with vehicle (DMSO), afatinib (29 nM) and pyrotinib (180 nM) on day 16 after dosing.