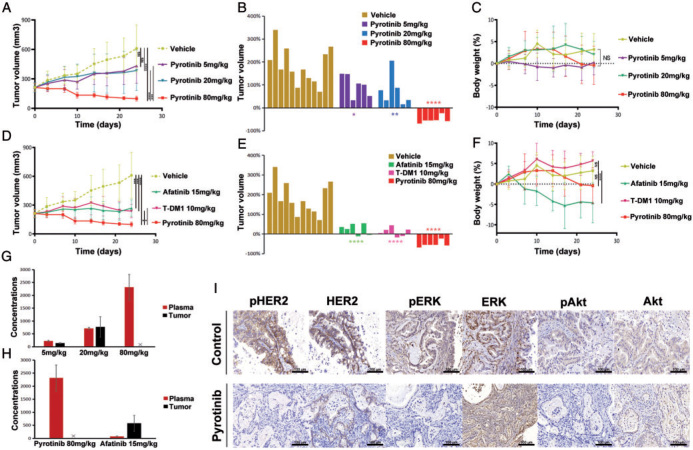
Figure 2.
In vivo activity of pyrotinib in PDX models. (A) Tumor volume curves of the PDX models treated with vehicle, and different doses of pyrotinib. (B) Tumor volume changes among mice treated with vehicle, and different doses of pyrotinib by 24 days. (C) Body weight changes among mice treated with vehicle, and different doses of pyrotinib. (D) Tumor volume curves of the PDX models treated with vehicle, pyrotinib, afatinib and T-DM1. (E) Tumor volume changes among mice treated with vehicle, and pyrotinib, afatinib and T-DM1 by 24 days. (F) Body weight changes among mice treated with vehicle, pyrotinib, afatinib and T-DM1. (G) Concentrations in plasma and in tumor in different doses of pyrotinib treatment groups were detected on day 24 after administration. The pyrotinib concentration in the tumor of the 80 mg/kg group was not available due to a low volume of tumor tissue after 24 days of pyrotinib treatment. (H) Concentrations in plasma and in tumor of pyrotinib and afatinib treatment groups were detected on day 24 after administration. (I) IHC staining of HER2 and its downstream proteins including ERK and Akt, in vehicle and pyrotinib treatment groups. *P < 0.05; **P < 0.01; ***P < 0.001; ****P < 0.0001.