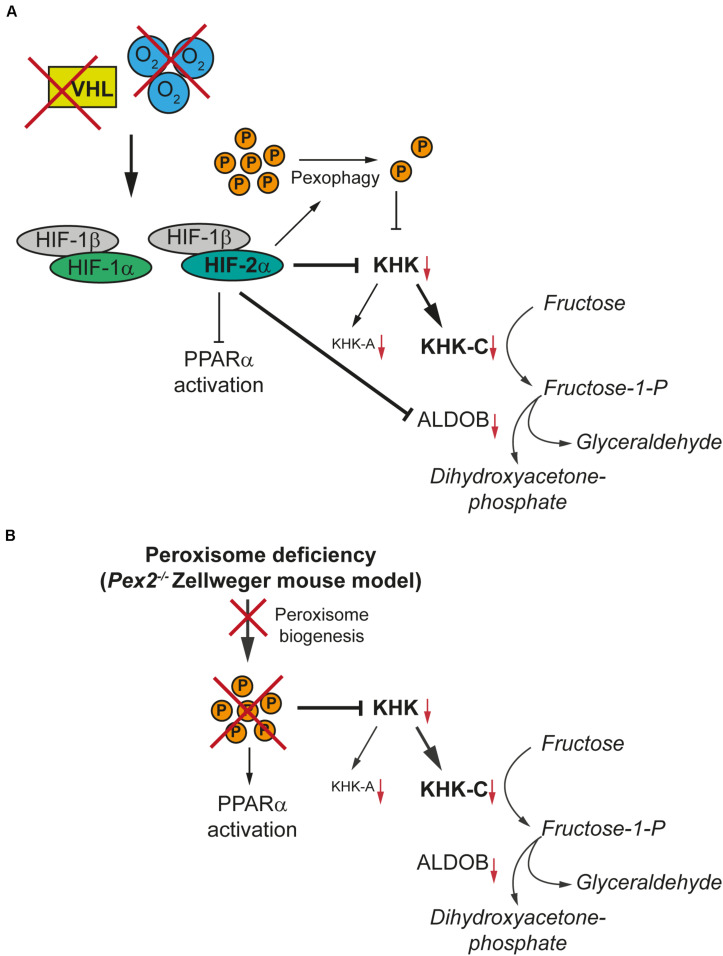
FIGURE 10.
HIF-2α signaling and peroxisome-deficiency reduce KHK expression in tissues with high fructolytic activity. KHK-C is the rate-limiting enzyme of fructolysis and the predominant isoform in tissues with high fructolytic activity, such as the liver and the kidney. (A) Hypoxia or loss of pVHL results in the stabilization of HIF-1α and HIF-2α. HIF-2α signaling induces the degradation of peroxisomes (P) via pexophagy and thereby reduces the number of peroxisomes. HIF-2α suppresses total Khk and associated Khkc and Khka expression as well as Aldob expression in tissues with high fructose metabolism such as the liver. The suppression of fructolytic genes by HIF-2α is not dependent on a decrease in the number of peroxisomes by HIF-2α-induced pexophagy. (B) The lack of functional peroxisomes due to a defect in peroxisome biogenesis also suppresses total Khk, Khkc, and Khka expression in the liver and kidney of the Pex2–/– Zellweger syndrome mouse model. The absence or a reduced number of peroxisomes and hence decreased peroxisomal metabolic activity leads to the accumulation of very long-chain fatty acids and very long-chain polyunsaturated fatty acids which are potent activators of PPARα. In contrast, HIF-2α signaling represses the ligand-dependent activation of PPARα, which would be a consequence of the decrease in peroxisome number caused by pexophagy. However, PPARα has only a minor effect on fructolysis.