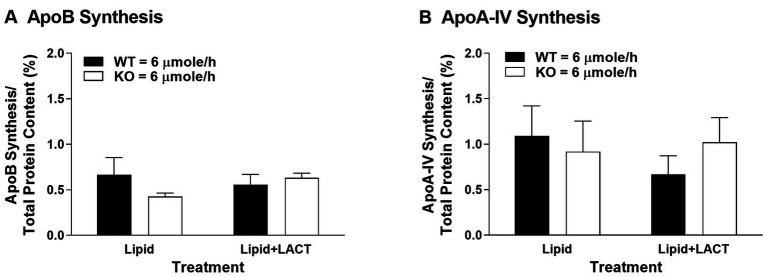
Figure 1.
Apolipoprotein (Apo) B (A) and ApoA-IV (B) protein synthesis in wildtype (WT) and Apobec-1 knockout (KO) mice. Chow-fed WT and Apobec-1 KO mice received an intraduodenal infusion of lipid emulsion with 6 μmoles/h of triolein for 4 h, then a 7.5 cm-segment of proximal jejunum isolated by ligature in anesthetized mice was incubated with 100 μl of saline mixture containing 3H-leucine (200 μCi per mouse) in the absence (Lipid) or presence of lactacystin (10 μM; Lipid + LACT) based on published protocols (Kalogeris et al., 1994; Liao and Chan, 2000). After 10 min of incubation, the segment was removed and washed with saline, and mucosa of the segment was scraped, homogenized in lysis buffer, and centrifuged. For total protein content of newly synthesized proteins, precipitation of 25 μl aliquots of the cytosolic homogenate was performed with trichloroacetic acid (TCA). For synthesis of ApoB and ApoA-IV, 25 μl aliquots of the cytosolic homogenate were electrophoresed through a 4–20% gradient acrylamide SDS gel (Bio-Rad Laboratories, Hercules, CA), the gel stained with Coomassie Blue and the band of ApoB or ApoA-IV excised. Radioactive amounts of the total protein precipitates as well as the bands of ApoB and ApoA-IV were determined using scintillation counting. Synthesis of ApoB and ApoA-IV was calculated by radioactive amount of ApoB or ApoA-IV divided by radioactive amount of total protein content and multiplied by 100 (%); since ApoB100 has twice the number of leucines for labeling in comparison to ApoB48 (525 vs. 261, respectively), the radioactive values for ApoB100 were divided by two before dividing by radioactive amount of total protein content in order to be able to compare the ApoB results on a per molecule basis. Data are expressed as mean ± SEM for seven or eight animals per group. Statistical significance was assessed by two-way ANOVA (no significant differences were seen at alpha = 0.05).