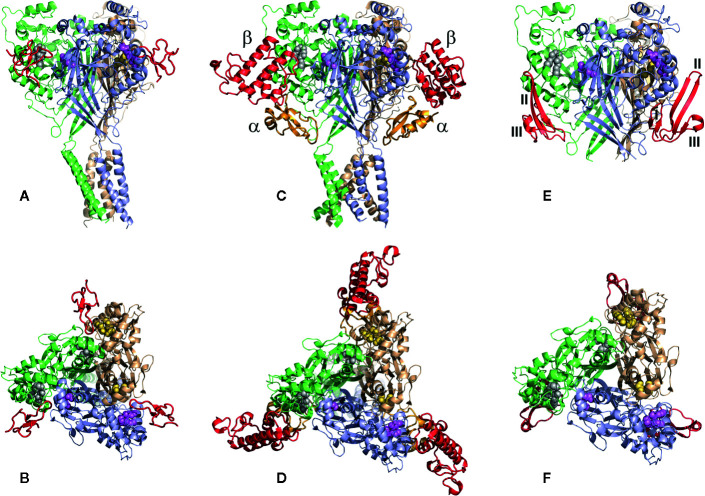
Figure 10.
The structures of the cASIC1a complexes with different peptide toxins. The channel subunits are shown by wheat, pale green, and blue colors. Asp and Glu residues forming the acidic pockets in corresponding subunits are shown by spheres colored in yellow, gray, and violet. (A, B) The side and top views of the complex cASIC1a/PcTx1 (PDB code 4FZ0). Toxins are shown using a red color. PcTx1 inserts its loop into the acidic pocket, simultaneously interacting with the finger and thumb domains of one channel subunit and the palm domain of adjacent subunit. (C, D) The side and top views of the complex cASIC1a/MitTx (PDB code 4NTW). Toxins’ α and β subunits are colored light orange and red, respectively. α-Subunit interacts with the wrist region and β1-β2 and β11-β12 linkers, whereas the β-subunit binds to the thumb and finger domains without penetration into the acidic pocket. (E, F) The model of the complex cASIC1a/mambalgin-1 is built based on the cryo-EM density map (Sun et al., 2018), crystal channel structure (PDB code 4FZ1), and crystal mambalgin-1 structure (PDB code 5DU1). Mambalgin-1 is shown using a red color. Loops of the polypeptide are numbered, and the mambalgin-1 form the contacts with the thumb domain by the first and second loops without penetration into the acidic pocket.