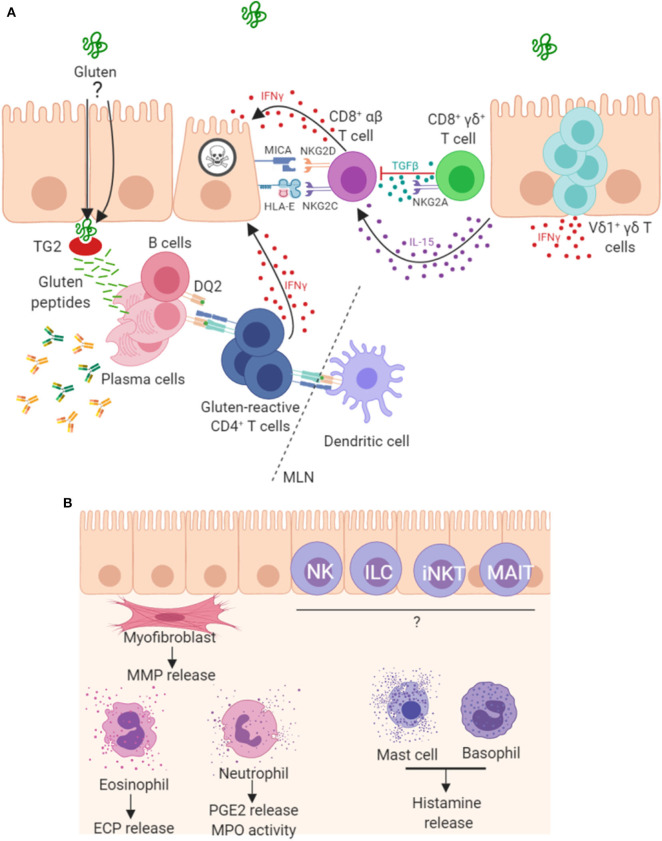
Figure 2.
Mechanisms of pathogenesis in coeliac disease. (A) It is well established that peptides derived from gluten are modified by TG2 and presented by antigen presenting cells in mesenteric lymph nodes (MLN) to CD4+ T cells in the context of HLA-DQ2. The resulting TH1 type response results in IFNγ production and intestinal inflammation. Chronic inflammation leads to expansion and persistence of Vδ1+γδ T cells, which also contribute to IFNγ production. Gluten peptides induce expression of IL-15 and stress molecules on enterocytes. The increased levels of IL-15 promote a NK-like phenotype in CD8+ T cells, contributing directly to enterocyte death. A proportion of CD8+γδ+ T cells are thought to play a regulatory role through secretion of TGF-β. Plasma cells are also abundant in the lesion where many express the immunodominant gluten peptide DQ2.5-glia-α1a and are induced to secrete antibodies that bind to TG2 and other targets. (B) Other less well-characterized mechanisms may play a role in lesion development. Intestinal myofibroblasts contribute to tissue remodeling by the secretion of matrix metalloproteases (MMPs) and via their contractile properties. These cells strongly express TG2 and α-actin. Innate-like lymphocytes including natural killer (NK cells), innate lymphoid cells (ILC), invariant natural killer T cells (iNKT) and mucosal-associated invariant T (MAIT) cells may all contribute to the lesion. Granulocytes, including eosinophils, neutrophils and basophils, and also mast cells have been detected in higher levels and may be involved in disease pathogenesis.