Figure 5. The Mif2 N‐terminus is prevented from high‐affinity Mtw1c binding in the full‐length molecule.
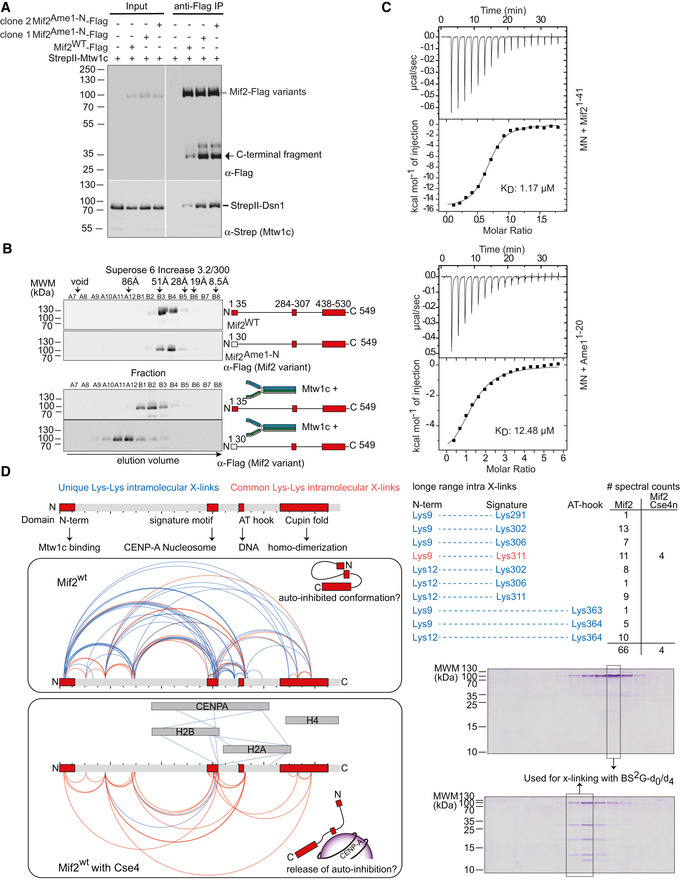
- Co‐purification of Strep‐Mtw1c through Flag‐Mif2 after co‐expression in insect cells. Proteins were detected by Western blotting using their respective tags.
- Western blotting of elution fractions from an SEC experiment of Mif2‐wt or Mif2Ame1‐N swap in the absence (upper panel) or presence (lower panel) of Mtw1c.
- ITC analysis of Mif21‐41 or Ame11‐20 binding to MN. Experiments were performed 3 or 4 times, respectively. One representative binding curve is shown, whereas the KD value represents the average of all experiments.
- Cross‐link (XL) MS analysis of the Mif2wt protein in isolation or bound to Cse4n using the cross‐linker Bis[sulfosuccinimidyl] glutarate. Mif2wt alone or in combination with Cse4n was subjected to analytical size‐exclusion chromatography at 10 μM concentration. The corresponding Coomassie‐stained SDS–PAGE gels of Mif2‐wt alone or in combination with Cse4n are shown. Fractions used for cross‐linking are highlighted. Topological map of Mif2 based on the identified intramolecular cross‐links. Common cross‐links that are found in both samples are shown in red, whereas unique cross‐links that can be only found in one of the samples are highlighted in blue. Conserved regions and their described functions are indicated. A list of cross‐links that link N‐terminal residues to C‐terminal regions of Mif2 is shown for Mif2 alone and bound to Cse4n leading to a proposed conformation of Mif2.
Source data are available online for this figure.
