Abstract
Protecting immunosuppressed patients during infectious disease outbreaks is crucial. During this novel coronavirus disease 2019 pandemic, preserving “clean areas” in hospitals assisting organ transplant recipients is key to protect them and to preserve transplantation activity. Evidence suggests that asymptomatic carriers might transmit the SARS‐CoV‐2, challenging the implementation of transmission preventive strategies. We report a single‐center experience using universal SARS‐CoV‐2 screening for all inpatients and newly admitted patients to an Organ Transplant Unit located in a region with significantly high community‐based transmission.
Keywords: COVID‐19, SARS‐CoV‐2, screening, transplantation
1. INTRODUCTION
The novel coronavirus disease 2019 (COVID‐19) emerged in Wuhan, China, in December 2019 and rapidly spread to many countries around the globe. On February 25, 2020, Brazil had its first tested positive patient and, on March 16, the first patient was diagnosed in our State (Ceara, located in the Northeast of the country), which is currently the third Brazilian State with the highest number of cases. 1
Infected people usually present mild upper respiratory symptoms, favoring quickly spread of the virus. Each infected patient transmits the SARS‐CoV‐2 to 2‐3 healthy persons. 2 It is unclear whether asymptomatic people shed SARS‐CoV‐2, potentially transmitting the virus. Previous studies demonstrated that asymptomatic carriers can transmit MERS‐CoV, 3 and similar transmission pattern has been suggested for SARS‐CoV‐2. 4 , 5 , 6
Given the unknown contribution of an asymptomatic carrier to spread the COVID‐19 and the paucity of diagnostic tests in most countries, screening strategies are not widely performed. We will describe the experience of a single center of screening all inpatients and newly admitted patients to the Organ Transplant Unit. The strategy was motivated by the occurrence of possible cases of nosocomial transmission. From this event, all newly admitted patients have been tested for COVID‐19 and maintained isolated from other patients until the test result is known.
2. CASES REPORT
On March 31, 2020, a 43‐year‐old man with alcoholic liver cirrhosis, hospitalized since March 23rd presented acute dyspnea and fever and was tested positive for SARS‐CoV‐2 (patient 1). He had severe chronic liver failure and had recently been hospitalized for similar cirrhosis symptoms. On the same day, a health professional (surgical resident) who made her rotation in the Transplant Unit in March, started flu‐like syndrome. On April 3rd, she was also tested positive.
Considering the possibility of nosocomial transmission and concerned about transmission to other inpatients, we decided to screen for COVID‐19 all inpatients who have potentially contacted the patient 1, the infected surgical resident and patients who have contacted them. In this initial step, three patients without COVID‐19 symptoms were screened and one tested positive (patient 2).
From then on (April 6, 2020), universal masking of all providers and patients at all times were implemented and all newly admitted patients to the Transplant Unit have been screened for COVID‐19 mild signs and symptoms and a nasopharyngeal swab specimen is obtained for qualitative reverse‐transcription polymerase chain reaction (RT‐PCR), whose turnaround time is two to 3 days (commercial kits: Bio‐Manguinhos/FIOCRUZ; Integrated DNA Technologies, Inc; and XGEN MASTER, Mobius Life Science).
Until RT‐PCR result, patients are kept in a specific ward and isolation precautions are adopted: Surgical masking for patients and their escorts is mandatory; healthcare providers should wear surgical mask, disposable apron, and face/eye protection. If aerosol‐generating procedures are planned, N95 respirator, disposable gloves, disposable mob cap, and disposable plastic apron are also required. Importantly, visits to inpatients are prohibited since March 20, 2020, and the medical bulletin is daily communicated by phone. Healthcare workers who contacted the surgical resident and/or patient 1 were advised to self‐monitoring for fever and other COVID‐19 symptoms and avoid work when ill. This recommendation was extended to all staff.
To date, twelve newly admitted patients without COVID‐19 symptoms were screened and six tested positive (patients 3 to 8). All positive patients have been immediately transferred to hospital units dedicated to COVID‐19 care. Current hospital protocol recommends that, if hospitalization lasts more than 14 days after the positive RT‐PCR test result, those patients could return to the Transplant Unit after two negative RT‐PCR tests.
Table 1 details and Figure 1 illustrates the clinical information on the infected individuals. Only one screened patient developed unequivocal COVID‐19 symptoms (Patient 2). Eight days after the positive RT‐PCR test, she presented acute respiratory distress syndrome (ARDS) symptoms (cough, fever, and dyspnea) requiring intensive care, mechanical ventilation, and vasoactive drugs. On April 21st, she died of refractory shock.
Table 1.
Clinical characteristics and outcomes of patient 1 and screened patients
| Patient ID | Baseline clinical condition |
Gender, age (years old) |
Reason for hospital admission | Admission date | RT‐PCR date | Known contact with infected patient | COVID‐19 symptoms | COVID‐19 treatment | Outcomes |
|---|---|---|---|---|---|---|---|---|---|
| 1 |
CLD LT WL |
Male, 43 | Severe chronic liver failure | February 23, 2020 | March 31, 2020 | Yes; HP | ARDS on Mar 31, 2020 | HCQ + AZ | Death on April 14 due to respiratory failure and septic shock |
| 2 |
CLD LT WL |
Female, 55 | Severe chronic liver failure | March 5, 2020 | April 3, 2020 |
Yes; HP and patient 1 |
ARDS on April 11, 2020 | HCQ + AZ | Death on April 21st due to refractory shock |
| 3 |
CLD LT WL |
Male, 64 | Severe chronic liver failure | April 6, 2020 | April 7, 2020 | No | No | No | Hospital discharge on April 21st |
| 4 | KT (Mar 2020) | Male, 37 | Acute pyelonephritis | April 8, 2020 | 10th Apr, 2020 | No | No | No | Hospital discharge on April 14th. No COVID‐19 symptoms until April 30th |
| 5 | KT (Dec 2013) | Female, 9 | Allograft dysfunction | April 8, 2020 | April 14, 2020 | No | No | No | Transferred to another hospital on April 20th |
| 6 | KT (Jun 2016) | Male, 76 | Urinary tract infection | April 23, 2020 | April 25, 2020 | No | No | No | Hospital discharge on May 20th. No COVID‐19 symptoms until May 9th |
| 7 | KT (Sep 2002) | Male, 40 | Deep vein thrombosis | April 27, 2020 | April 30, 2020 | No | No | No | Hospital discharge on May 4th. No COVID‐19 symptoms until May 14th |
| 8 | KT (May 2013) | Male, 34 | Graft loss and return to dialysis (CAD) | April 27, 2020 | April 30, 2020 | No | No | No | Hospital discharge on May 2nd. No COVID‐19 symptoms until May 14th |
Abbreviations: ARDS, acute respiratory distress syndrome; AZ, azithromycin; CAD, chronic allograft dysfunction; CLD, chronic liver disease; CMV, cytomegalovirus; COVID‐19, coronavirus disease 2019; HCQ, hydroxychloroquine; HP, healthy professional; KT, kidney transplant; LT, liver transplant; RT‐PCR, reverse‐transcription polymerase chain reaction; WL, waiting list.
FIGURE 1.
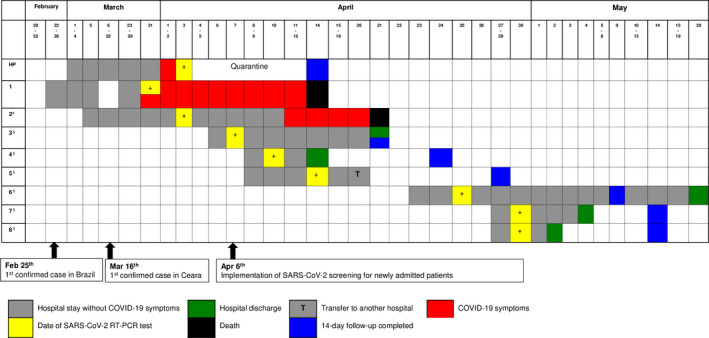
Timeline from hospitalization to outcome of COVID‐19 in screened patients. Legend: HP, healthy professional; #patient who contacted the HP or the patient 1; §newly admitted patients
Patients 3‐8 were monitored during hospitalization and, after the discharged from the hospital, they were remotely monitored for 14 days after RT‐PCR result, and no COVID‐19 symptom was detected.
3. DISCUSSION
We reported the COVID‐19 screening strategy adopted by our center in a attempt to prevent nosocomial transmission and keep “clean” the Transplant Unit. This report aims to bring into the light important unanswered questions on COVID‐19 and to make some hypotheses.
It is known that COVID‐19 is a highly contagious disease. The reported basic reproductive number (R0) is 2.2, which means that, on average, one case generates 2‐3 additional cases, resulting in an exponential rate of infected patients. The scarce available evidence suggests that this high transmissibility potential is a result of: (a) prolonged virus survival on surfaces; (b) transmission by respiratory and aerosolized droplets; (c) relatively low mortality, preserving the host; and (d) the possibility of transmission by asymptomatic and presymptomatic people. 2 , 7 The mechanism by which asymptomatic carriers could transmit the coronavirus is still understood. However, ensuring the complete absence of symptoms is a challenge. In addition, new non‐respiratory symptoms, which can initially be attributed to other pathologies, have recently been associated with COVID‐19, such as abdominal pain, glomerulopathy, cutaneous lesions, conjunctivitis, encephalitis, hepatitis, myocarditis, and thromboembolic manifestations. 8 , 9 , 10 , 11
Researchers at Columbia University Irving Medical Center, New York, recently reported their experience using universal screening for obstetrical population admitted to the hospital for delivery. From 215 interviewed pregnant women, 4 (1.9%) presented signs/symptoms and were tested positive. From asymptomatic ones, the quantitative RT‐PCR was positive in 29 (13.7%). 12 As a limitation of our strategy, our RT‐PCR test was qualitative and we could not estimate the magnitude of viral load, which implies the risk of transmission and possibly the chance of developing symptoms.
In the context of organ transplantation, there are a lot of unanswered questions. It was suggested that lymphopenia is associate with severe clinical symptoms and death, 13 but we do not know whether the immunosuppressive drug‐induced modulation on immune and inflammatory response can modify the clinical, laboratory, and radiographic presentation, as well as the outcomes. We also do not know if asymptomatic carrier transplant recipients have higher risk to develop symptoms when compared to the general population.
Evidence points out that the shedding of viral RNA from sputum outlasts the end of symptoms. 14 Similar to other viral infections, it is possible that viral shedding is more prolonged in transplant recipients, suggesting that more than one swab is necessary to discontinue the isolation precautions. 15 , 16
We opted not treating those patients unless they develop COVID‐19 symptoms. In fact, no preventive strategy is available for contacts and for SARS‐CoV‐2 carriers.
The aim of this report is not to identify an index case, nor correlate patient with a source of contagion, but to demonstrate that screening is possibly a good strategy to preserve “clean units” assigned to immunosuppressed patient care, maintaining transplantation activity more safely. The universal screening might guide hospital isolation practices and bed assignments, and the proper use of personal protective equipment (PPE). 12
Since the sensitivity of RT‐PCR is not 100%, active and rigorous clinical investigation of subtle signs and symptoms in this population is also essential. 17 In addition, recommendations for biosafety and hospital infection control should be strictly followed, including the use of PPE and hands hygiene.
CONFLICT OF INTEREST
The authors of this manuscript have no conflicts of interests to disclosure.
AUTHOR CONTRIBUTIONS
TVS‐F, IRCB, and RME involved in concept/design. TVS‐F, MSSNL, and LATJC collected data. TVS‐F, MLMBO, MSSNL, IRSP, and LATJC analyzed/interpreted data. TVS‐F drafted the article. TVS‐F, IRCB, MLMBO, MSSNL, IRSP, LATJC, and RME involved in critical revision of article/approval of article.
ACKNOWLEDGEMENTS
Authors would like to thank the health professional team of our Transplant Unit for the dedication and resilience during this pandemic.
de Sandes-Freitas TV, Canito Brasil IR, de Oliveira Sales MLMB, et al. Lessons from SARS-CoV-2 screening in a Brazilian organ transplant unit. Transpl Infect Dis. 2020;22:e13376. 10.1111/tid.13376
REFERENCES
- 1. Boletim n. 13, Centro de Operações de Emergência em Saúde Pública / Doença pelo Coronavirus 2019 (COE‐COVID19), Health Ministry, Brazil Report 13, Epidemiological Week 17. https://coronavirus.saude.gov.br/boletins‐epidemiologicos. Accessed May 28th, 2020.
- 2. Ye Q, Wang B, Mao J, et al. Epidemiological analysis of COVID‐19 and practical experience from China. J Med Virol. 2020;92(7):755‐769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Grant R, Malik MR, Elkholy A, et al. A review of asymptomatic and subclinical middle east respiratory syndrome coronavirus infections. Epidemiol Rev. 2019;41(1):69‐81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Pan X, Chen D, Xia Y, et al. Asymptomatic cases in a family cluster with SARS‐CoV‐2 infection. Lancet Infect Dis. 2020;20(4):410‐411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Rothe C, Schunk M, Sothmann P, et al. Transmission of 2019‐nCoV infection from an asymptomatic contact in Germany. N Engl J Med. 2020;382(10):970‐971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Tong ZD, Tang A, Li KF, et al. Potential presymptomatic transmission of SARS‐CoV‐2, Zhejiang Province, China, 2020. Emerg Infect Dis. 2020;26(5):1052‐1054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Li Q, Guan X, Wu P, et al. Early transmission dynamics in Wuhan, China, of novel coronavirus‐infected pneumonia. N Engl J Med. 2020;382(13):1199‐1207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Poyiadji N, Shahin G, Noujaim D, et al. COVID‐19‐associated acute hemorrhagic necrotizing encephalopathy: CT and MRI features. Radiology. 2020: 201187. 10.1148/radiol.2020201187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Xiao F, Tang M, Zheng X, et al. Evidence for gastrointestinal infection of SARS‐CoV‐2. Gastroenterology. 2020;158(6):1831‐1833.e3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Larsen CP, Bourne TD, Wilson JD, et al. Collapsing glomerulopathy in a patient with coronavirus disease 2019 (COVID‐19). Kidney Int Rep. 2020;5(6):935‐939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Lu S, Lin J, Zhang Z, et al. Alert for non‐respiratory symptoms of coronavirus disease 2019 (COVID‐19) patients in epidemic period: a case report of familial cluster with three asymptomatic COVID‐19 patients. J Med Virol. 2020. 10.1002/jmv.25776. [DOI] [PubMed] [Google Scholar]
- 12. Sutton D, Fuchs K, D'Alton M, et al. Universal screening for SARS‐CoV‐2 in women admitted for delivery. N Engl J Med. 2020;382(22):2163‐2164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Guan WJ, Ni ZY, Hu Y, et al. Clinical characteristics of coronavirus disease 2019 in China. N Engl J Med. 2020;382(18):1708‐1720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Zhou B, She J, Wang Y, et al. The duration of viral shedding of discharged patients with severe COVID‐19. Clin Infect Dis. 2020;395(10229):1054‐1062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Xu K, Chen Y, Yuan J, et al. Factors associated with prolonged viral RNA shedding in patients with COVID‐19. Clin Infect Dis. 2020: ciaa351. 10.1093/cid/ciaa351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Hijano DR, Maron G, Hayden RT. Respiratory viral infections in patients with cancer or undergoing hematopoietic cell transplant. Front Microbiol. 2018;9:3097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Wang Y, Kang H, Liu X, et al. Combination of RT‐qPCR testing and clinical features for diagnosis of COVID‐19 facilitates management of SARS‐CoV‐2 outbreak. J Med Virol. 2020;92(6):538‐539. [DOI] [PMC free article] [PubMed] [Google Scholar]


