Abstract
Aim
The aim of this work was to investigate the sensitivity and utility of CT of the chest in diagnosing active SARS‐Cov‐2 (COVID‐19) infection, and its potential application to the surgical setting.
Method
A literature review was conducted using Google Scholar® and MEDLINE®/PubMed® to identify current available evidence regarding the sensitivity of CT chest compared with RT‐PCR for the diagnosis of COVID‐19‐positive patients. GRADE criteria and the QUADAS 2 tool were used to assess the level of evidence.
Results
A total of 20 articles were identified that addressed the question of sensitivity of CT for diagnosis of symptomatic and asymptomatic COVID‐19‐positive patients. Overall sensitivity of CT scan ranged from 57%–100% for symptomatic and 46%–100% for asymptomatic COVID‐19 patients, while that of RT‐PCR ranged from 39%–89%. CT chest was a better diagnostic modality and capable of detecting active infection earlier in the time course of infection than RT‐PCR in symptomatic patients. In asymptomatic patients, disease prevalence seems to play a role in the positive predictive value. Minimal evidence exists regarding the sensitivity of CT in patients who are asymptomatic.
Conclusions
In surgical patients, CT chest should be considered as an important adjunct for detection of COVID‐19 infection in patients who are symptomatic with negative RT‐PCR prior to any operation. For surgical patients who are asymptomatic, there is insufficient evidence to recommend routine preoperative CT chest for COVID‐19 screening.
Keywords: COVID‐19, CT Chest, RT‐PCR, Sensitivity
Introduction
Since December 2019, severe acute respiratory syndrome coronavirus 2 (SARS‐CoV‐2) has quickly spread worldwide, leading to a global pandemic (COVID‐19), and drastically altering everyday life and clinical practices. Its high rate of transmission, with a basic reproduction number (R 0) estimated to be between 2 and 3, and its resultant rate of global spread are alarming [1, 2]. The overall mortality rate has been quoted to be between 2.3% and 7.3% [3, 4].
Studies have demonstrated that patients who are older and those with increased comorbidities, including hypertension, diabetes, coronary artery disease, underlying respiratory conditions and obesity, are at a higher risk of adverse outcomes and mortality if infected with SARS‐CoV‐2 [5, 6]. Estimates show that the in‐hospital intubation rate is close to 20%, with roughly 80% of intubated patients ultimately dying, demonstrating an overall in‐hospital mortality rate of 16% [5, 6, 7, 8]. The perioperative morbidity and mortality associated with elective surgery are also higher among COVID‐19‐positive patients [5, 8, 9, 10]. In a recently published international study of 1128 COVID‐19‐positive patients undergoing emergency (74.0%) and elective (24.8%) surgery, the overall 30‐day mortality was 23.8%, with 51.2% of patients having a pulmonary complication [10]. Of the patients who had a pulmonary complication, the mortality rate was 38.0% [10]. Therefore, identifying COVID‐19‐positive patients prior to elective surgery and delaying nonemergent operations until patients recover from their acute infection will decrease potential morbidity and mortality in surgical patients.
Adequate testing, early diagnosis, isolation and contact tracing have been the key to containing the spread of SARS‐CoV‐2 [11]. Currently, nasopharyngeal reverse transcriptase polymerase chain reaction (RT‐PCR) is the most common way of testing for COVID‐19. Problematic issues with these tests, however, include an approximately 30% false‐negative rate, with results often taking many days to be reported, and the lack of availability of the tests themselves [12, 13]. However, characteristic findings on CT chest scans may also serve as a means of diagnosis. CT scans typically demonstrate peripheral and subpleural lesions (96.1%), with ground‐glass opacities and consolidations (96.1%), and disease seen in all five lobes (74.5%) [14, 15, 16]. Importantly, Pan et al. demonstrated that CT findings can change depending on the stage of SARS‐CoV‐2 infection [17]. Initially, within the first 4 days of symptom onset, 75% of patients have characteristic findings of ground‐glass opacities, 25% a 'crazy‐paving pattern' and 42% consolidation. As the infection progresses, repeat CT chest demonstrates further involvement in a bilateral multilobe distribution [5, 6, 7, 8], increasingly prominent consolidation (9–13 days) and finally ground‐glass opacities with regression of the crazy‐paving pattern initially observed (> 14 days after initial onset).
The aim of this review was to examine available evidence that exists for evaluating CT chest as a diagnostic tool compared with the current standard of care, RT‐PCR, for COVID‐19 diagnosis for all symptomatic and asymptomatic patients. Clarifying the role and utility of CT chest will be important, as screening asymptomatic patients may lead to changes in the current screening algorithms for presurgical patients. Due to the high perioperative morbidity and mortality in COVID‐19‐positive patients, identifying COVID‐19 patients accurately and quickly will be an important consideration prior to surgery.
Method
A PRISMA‐compliant systematic review of the literature was conducted to evaluate available evidence regarding the sensitivity and general utility of CT chest compared with RT‐PCR in COVID‐19 diagnosis [18]. A PRISM flowchart of the selection of relevant studies can be found in Fig. 1. The PICO (Population, Intervention, Comparison and Outcomes) question formulated was: what is the sensitivity of CT chest compared with RT‐PCR for diagnosis of COVID‐19 asymptomatic and symptomatic patients?
Figure 1.
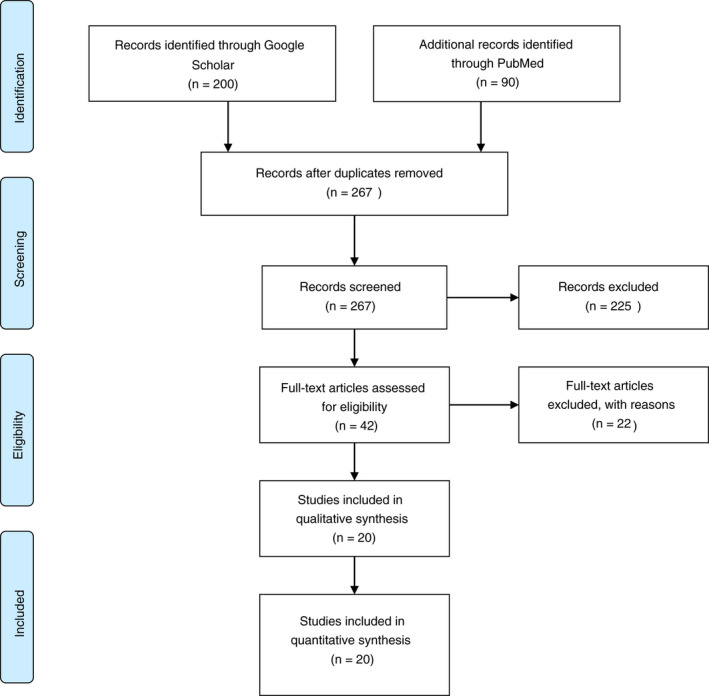
PRISMA flowchart.
Search strategy and study selection
Google Scholar® and MEDLINE®/PubMed® were used to search for primary articles evaluating the use of CT chest for evaluation, screening and diagnosis of COVID‐19. Due to the limited data available and the acceptance of fast‐tracked publications on COVID‐19, some studies included are unpublished manuscripts or manuscripts submitted for publication. Studies from 1 December 2019 until 7 June 2020 were included. The MEDLINE®/PubMed® database was queried for the terms ‘CT chest’, ‘RT‐PCR’ and ‘COVID 19’. Additionally, Google Scholar® was queried with the terms ‘CT chest’ and ‘RT‐PCR’ and ‘COVID 19’ and ‘sensitivity’. All 90 results from MEDLINE®/PubMed® and the top 200 results from the Google Scholar® search were examined and studies that met the inclusion criteria were included in the analysis.
Inclusion criteria
The inclusion criteria for analysis of studies are as follows: (1) study population of any patient (symptomatic or asymptomatic) undergoing testing for COVID‐19 infection consisting of more than five patients; (2) studies primarily using RT‐PCR as a standard method of detecting COVID‐19 infection; (3) studies in which diagnostic CT chest was performed in addition to RT‐PCR; (4) studies in which sensitivity and/or specificity of either study was recorded. Exclusion criteria consisted of eliminating case reports or series of five or fewer patients and studies dealing with other aspects of CT chest in diagnosis of COVID‐19 that did not specifically address comparison with RT‐PCR.
Data assessment and determination of quality
The authors reviewed data from the studies with type of study, total patient population and sensitivities. The main outcome assessed in this review was an examination of the sensitivity of CT chest imaging in diagnosing COVID‐19 infection in both symptomatic and asymptomatic COVID‐19‐positive patients compared with RT‐PCR. Quality of the studies was judged based on the GRADE Working Group guidelines [19] and QUADAS 2 for diagnostic studies [20].
Results
Figure 1 shows a PRISMA flow chart demonstrating study selection and the studies that were ultimately included in our review [18]. A total of 20 studies were identified that fitted the inclusion criteria (Tables 1 and 2). The studies examined were grouped by patient symptoms in relation to diagnostic testing with RT‐PCR and CT chest.
Table 1.
Primary studies: COVID‐19 symptomatic patients.
| Study/country | Type of study | n | Mean age (years) ± SD | Gender (% male) | Reported sensitivity of CT chest (95% CI if available) |
|---|---|---|---|---|---|
| Ai et al., China [26] | Retrospective | 1014 | 51 ± 15 years | 46 | 97% (95–98%) |
| Bai et al., China/USA [29] | Retrospective | 219 | 44.8 ± 14.5 | 54 | 67–94% |
| Bernheim et al., China [15] | Retrospective | 121 | 45.3 | 50 | 88%, as measured 'late' in disease |
| Caruso et al., Italy [33] | Prospective | 158 | 57 ± 15 | 52 | 97% (88–99%) |
| Chen et al., China [25] | Retrospective | 21 | 49 ± 15.7 | 57 | 95% |
| Dangis et al., Belgium [31] | Retrospective | 192 | 61 ± 18.2 | 45 | 86.7% |
| Fang et al., China [27] | Retrospective | 51 | 45 | 57 | 98% (90–100%) |
| Fu et al., China [32] | Retrospective | 64 | 46.1 ± 13.1 | 45 | 85.9% |
| Gietema et al., Netherlands [34] | Prospective | 193 | 66 | 58 | 89.2% |
| He et al., China [21] | Retrospective | 82 | 52 | 50 | 79% |
| Himoto et al., Japan [30] | Retrospective | 21 | 58.5 | 57 | 100% |
| Li et al., China [14] | Retrospective | 225 | 50 ± 14 | 53 | 86.2% |
| Miao et al., China [23] | Retrospective | 130 | 45.1 ± 13.4 | 52 | 57% |
| Pan et al., China [17] | Retrospective | 84 | 40 ± 9 | 29 | 100% when all time periods measured |
| Wang et al., China [24] | Retrospective | 114 | 53 | 49 | 96.5% |
| Wen et al., China [28] | Retrospective | 103 | 46 | 46 | 93% (85–97%) |
| Zhao et al., China [16] | Retrospective | 34 | 48 | 58 | 89.5% |
Table 2.
Primary studies: COVID‐19 asymptomatic patients.
COVID‐19 symptomatic patients
There were 17 primary studies found in the literature that assessed the sensitivity of CT chest compared with RT‐PCR in symptomatic COVID‐19‐positive patients with sensitivities ranging from 57% to 100% depending on the study (Table 1). An examination of these studies demonstrated that 15 studies were retrospective analyses with only two prospective studies identified.
The retrospective studies examined demonstrated a high sensitivity rate for CT chest compared with RT‐PCR in symptomatic COVID‐19 patients [15, 16, 17, 21, 22, 23, 24, 25, 26, 27, 28, 29, 30, 31, 32]. The largest study to date, by Ai et al., is a retrospective analysis which included 1014 patients who were being tested for COVID‐19 in Wuhan, China [26]. The study directly compared the efficacy of CT chest with RT‐PCR for diagnosis of suspected COVID‐19 patients presenting with clinical symptoms such as fever and cough [26]. RT‐PCR identified 59% of COVID‐19 patients while CT chest detected 88% of positive patients [26]. The sensitivity of CT chest was 97% (95% CI 95–98%) based on positive RT‐PCR results [26]. In patients who were RT‐PCR‐negative, 75% of these patients had CT scan findings consistent with positive COVID‐19 results. For RT‐PCR‐negative patients, patients were classified into highly likely, probable or uncertain based on follow‐up CT scans. The mean result time for RT‐PCR to turn from negative to positive was 5.1 ± 1.5 days, while CT scan results were more immediate with a higher sensitivity than RT‐PCR [26]. Specificity, however, was quoted to be 25% (95% CI 22–30%) in this study [26].
The importance of early timing of CT chest along with RT‐PCR was continually emphasized in the studies reviewed as CT chest had a higher sensitivity in symptomatic COVID‐19 patients with negative RT‐PCR early on in the infection course [27, 28]. Fang et al. found a similar lead time to Ai et al. in a retrospective review of 51 patients [26, 27]. In this study, patients were included who had either travelled to high‐risk areas and had contact with those with symptoms or were themselves symptomatic [27]. Ninety‐eight per cent (95% CI 90–100%) of patients with COVID‐19 had evidence of abnormal CT findings at an average of 3 ± 3 days from initial onset of disease, while RT‐PCR testing has been demonstrated to be only 71% (95% CI 56–83%) sensitive at 3 ± 3 days (P < 0.001) [27]. Sensitivity of RT‐PCR increased as sequential tests were done after initial testing – with 23.5% of patients requiring a second test, 3.9% requiring a third test and 2.0% requiring a fourth test [27]. Pan et al. [17] demonstrated that more than 75% of RT‐PCR‐positive patients displayed characteristic findings on CT within the first 4 days; in this study, however, they found the peak of findings on CT chest to occur 10 days after the onset of symptoms.
The two prospective studies included in this review were by Caruso et al. and Gietema et al [33, 34]. Caruso et al. enrolled 158 consecutive patients suspected to have COVID‐19 infection based on symptoms of fever, cough and dyspnoea [33]. All patients underwent RT‐PCR and CT chest to assess for infection, with 39% of patients being positive for RT‐PCR and 64% of patients having positive CT findings. These included ground‐glass opacities (100%), multilobe involvement (93%) and bilateral pneumonia (91%) [33]. Overall sensitivity, specificity and accuracy of CT scan for COVID‐19 pneumonia were 97% (95% CI 88–99%), 56% (95% CI 45–66%) and 72% (95% CI 64–78%), respectively [33]. Gietema et al. enrolled patients who presented to the Emergency Department over a 10‐day period with symptoms characteristic of COVID‐19 [34]. Patients were tested with both RT‐PCR and CT. CT was shown to have a sensitivity of 89.2% and was more likely to be predictive in those who were at high risk for pneumonia and with sepsis [34].
Specificity was examined by some, but not all, of the studies that were included in this analysis. The specificity reported varied widely by study and ranged from 24–100% [26, 29]. In Bai et al. [29], seven radiologists blindly reviewed the scans of 219 patients with a diagnosis of 'pneumonia', with the following results: a median specificity of 93%, with three of the radiologists demonstrating 100% specificity; the sensitivity in the same study ranged from 67–97%. Another study from Japan by Himoto et al. [30] of clinically symptomatic patients reported a specificity of 93% (95% CI 67–98%) when taking more specific CT scan characteristics into account, such as bilateral ground‐glass opacities and peripheral predominant lesions without airway abnormalities, nodules, mediastinal lymphadenopathy or pleural effusions.
COVID‐19 asymptomatic patients
Our systematic review identified three articles that met our inclusion criteria and compared the sensitivity of CT chest with RT‐PCR in asymptomatic COVID‐19‐positive patients (Table 2) [35, 36, 37]. The largest cohort of asymptomatic patients in this review comes from Inui et al. [35]. These authors looked at 104 confirmed RT‐PCR cases from the Diamond Princess cruise ship [35]. In this study, 76 of the patients were asymptomatic and 41 patients symptomatic [35]. The asymptomatic patients were less likely than the symptomatic patients to have abnormal findings on CT scan, with 54% having characteristic CT findings in the asymptomatic group and 79% having CT findings in the symptomatic group [35]. Another retrospective analysis identified 63 asymptomatic, RT‐PCR confirmed, COVID‐19‐positive patients through contact tracing with COVID‐19‐positive individuals [36]. Of the asymptomatic patients who underwent CT chest, 46% had abnormal CT chest findings [36]. In contrast, Shi et al. found that 15 of 15 asymptomatic, but RT‐PCR‐positive, patients in Wuhan, China displayed ground‐glass opacities on CT chest [37].
Discussion
Knowledge of diagnosis and treatment modalities of COVID‐19 is a rapidly evolving landscape as new information is obtained about the infection on a weekly basis. The advantage of CT scan for detecting COVID‐19 in symptomatic patients with a higher sensitivity and at an earlier time period of infection is important and should be further clarified in prospective studies [17, 27, 38, 39]. The potential implications of using CT scan as an adjunct for diagnosis and assessment of disease progression in symptomatic patients can be important in the diagnosis of RT‐PCR‐negative patients with COVID‐19 symptoms who require emergency or urgent surgery. Based on current evidence, CT scans have been shown to have a higher sensitivity early in the infection time course for symptomatic patients compared with conventional RT‐PCR, but its utility continues to remain uncertain in asymptomatic patients, especially regarding preoperative surgical patients. Identifying the role of CT imaging in diagnosis of COVID‐19 in symptomatic and asymptomatic patients can be important as it is a readily available tool in nearly all healthcare facilities in the world, and the results are immediately reported.
There appears to be a role for CT imaging beyond simply that of a resource‐constrained environment, as recently suggested by a multinational consensus from the Fleischner Society [40]. Given the high perioperative morbidity and mortality seen in several reviews of COVID‐19 patients undergoing surgery, even elective surgery [5, 8, 9, 10], and the increased rate of transmission to healthcare providers during aerosolized procedures, CT chest can be a useful preprocedural diagnostic adjunct to surgeons, endoscopists, anaesthetists and other procedural personnel prior to any aerosolizing procedures [16, 41, 42, 43, 44]. With this information, it may be recommended that a patient with any clinical symptoms of COVID‐19 should potentially undergo a CT chest in addition to RT‐PCR and typical CT abdomen/pelvis required prior to certain procedures (cancer, urgent or emergent abdominal operation, endoscopy, etc.). This will lead to the operations being performed in a timely fashion for those without findings on CT chest and decrease the overall hospital stay and cost. This rationale appears consistent with The Royal College of Radiologists' recent recommendations that patients who are suspected to have an abdominal emergency should undergo concurrent CT chest at the time of CT abdomen/pelvis [45].
CT chest protocols are varied between studies, but intravenous contrast medium is not necessary to make a diagnosis [27, 33]. The radiation doses needed for adequate detection of COVID‐19 were not recorded in many studies [16, 26, 27, 33], but the average CT dose index (CTDIvol) was 8.4 ± 2.0 mGy (range 5.2–12.6 mGy) for examining COVID‐19 patients [17] It has been suggested that reducing a patient’s radiation dose to one‐eighth or one‐ninth of the standard dose (0.203 mSv) will still allow for adequate imaging of lung parenchyma to identify SARS‐CoV‐2 infection [42]. Each standard‐dose CT scan (4–7 mSv) confers an oncological risk of 0.05–0.7%; by further decreasing that risk eight‐ to nine‐fold, the potential benefits far outweigh the potential adverse effect to patients [42, 46, 47].
Interestingly, CT scans can accurately predict not only a positive COVID‐19 infection in symptomatic patients, but also potentially assess the stage of infection and the time point and duration of infection [15, 17]. While RT‐PCR provides a qualitative answer, CT scans may allow clinicians a glimpse of the duration of infection with different findings at each stage [17]. While more information regarding the patterns of CT scans during the time course of infection is needed, this provides a context for a positive COVID‐19 test. CT chest can actually help clinicians to delineate the progression of the infective process in a patient, which could provide an optimal time frame for when an operation or procedure can safely be performed in patients requiring emergent or urgent operations. Given the high perioperative morbidity and mortality known thus far in COVID‐19‐positive patients, elective operations should be deferred in such patients regardless of symptoms [5, 8, 9, 10].
The role of a preoperative CT scan is certainly less clear in asymptomatic patients [35, 37, 38, 48]. The evidence suggests that there is less utility for CT scan in asymptomatic patients than in symptomatic patients [35, 37, 38, 48]. There is also relatively little evidence available specifically addressing management in asymptomatic presurgical patients. A meta‐analysis performed by Kim et al. demonstrated that the prevalence of disease also seemed to play a role in the positive predictive value (PPV), with a PPV of only 14.2% at a prevalence of 10%, compared with a PPV of 90.8% for RT‐PCR [39]. Additionally, they found that sensitivity is affected by the proportion of asymptomatic patients included and that in low‐prevalence countries the PPV of CT scan was ten times lower than that of RT‐PCR [39]. Whether or not CT chest will have any further value in addition to RT‐PCR in asymptomatic patients in preoperative (abdominal surgery) patients specifically is yet to be determined, and we should be cautious in extrapolating data from symptomatic patients to asymptomatic patients.
Clinical trials evaluating the role of CT chest in asymptomatic patients are under way in the Netherlands with formal results still pending [48]. Preliminary results of this prospective study from the Netherlands screening asymptomatic patients preoperatively using both CT chest and RT‐PCR prior to elective procedures demonstrated a total of 1.5–2.0% COVID‐19‐positive patients [48]. In this study, CT chest afforded an additional value of 0.1% in identifying patients that RT‐PCR potentially missed [48]. Final results from this study will be helpful in identifying if preoperative screening of asymptomatic patients with CT chest is necessary. Until more evidence is available in this rapidly changing landscape, it will remain unclear what the role of CT chest will be as a general screening tool for COVID‐19 infection in preoperative (abdominal surgery) patients.
The main limitation of this study is the quality of the evidence currently available. At this time, because of the retrospective nature of most of the studies regarding CT chest and RT‐PCR, there is a high selection bias. Almost all of the studies conducted thus far have been conducted in symptomatic patients with a few studies examining only asymptomatic patients. The overall grade of the recommendations of the papers reviewed is low, as most of the studies in this review consist of retrospective analyses [19]. Additional use of the QUADAS 2 assessment further demonstrates that there is a high risk for potential bias due to study design and patient selection. The concern regarding applicability of the evidence available thus far is low (Table 3 and Fig. 2). Further investigation regarding perioperative screening protocols in asymptomatic and symptomatic patients, along with better‐defined radiological criteria for detecting COVID‐19 and its progression, is still needed.
Table 3.
Assessment of quality of evidence using GRADE and QUADAS 2 tools.
| Study | Grade* | QUADAS 2† | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Risk of bias | Applicability concerns | ||||||||
| Patient selection | Index test | Reference standard | Flow and timing | Patient selection | Index test | Reference standard | |||
| Symptomatic COVID‐19 patient studies | Ai et al. [26] | Low | High | High | High | High | Low | Low | Low |
| Bai et al. [29] | Low | High | High | High | High | Low | Low | Low | |
| Bernheim et al. [15] | Very low | Low | High | Low | Low | Low | Low | Low | |
| Caruso et al. [33] | Moderate | Low | Low | Low | Low | Low | Low | Low | |
| Chen et al. [25] | Low | High | Low | Unclear | Low | Low | Low | Low | |
| Dangis et al. [31] | Low | High | Unclear | Unclear | Unclear | Low | Low | Low | |
| Fang et al. [27] | Low | Low | Unclear | Low | High | Low | Low | Low | |
| Fu et al. [32] | Low | High | High | Low | Unclear | Low | Low | Low | |
| Gietema et al. [34] | Moderate | Low | Low | Low | Low | Low | Low | Low | |
| He et al. [21] | Very low | Low | Low | Low | Low | Low | Low | Low | |
| Himoto et al. [30] | Very low | High | Low | Unclear | Unclear | Low | Low | Low | |
| Li et al. [14] | Low | Unclear | Unclear | Low | Unclear | Low | Low | Low | |
| Miao et al. [23] | Low | Low | Low | Low | Unclear | Low | Low | Low | |
| Pan et al. [17] | Low | High | Unclear | Low | Unclear | High | Low | Low | |
| Wang et al. [24] | Low | Low | Low | Low | Low | Low | Low | Low | |
| Wen et al. [28] | Low | Low | Low | Low | Unclear | Low | Low | Low | |
| Zhao et al. [16] | Very low | Low | Low | Low | Unclear | Low | Low | Low | |
| Asymptomatic COVID‐19 patient studies | Inui et al. [35] | Low | High | Low | Low | Low | High | Low | Low |
| Shi et al. [37] | Low | High | High | Low | High | Low | Low | Low | |
| Wang Y et al. [36] | Low | High | Unclear | Low | Unclear | High | Low | Low | |
Figure 2.
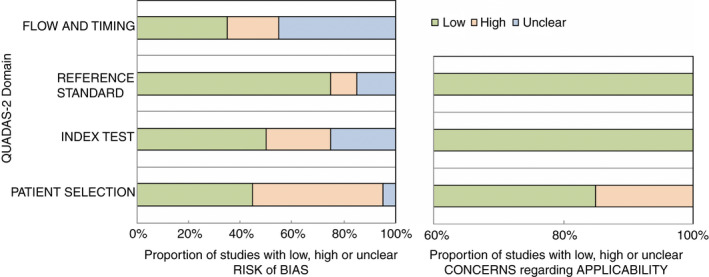
QUADAS 2 assessment.
Conclusion
CT chest can be a highly sensitive diagnostic test for any symptomatic COVID‐19‐positive patients and is capable of detecting COVID‐19 infection earlier in the infectious course than RT‐PCR. Based on preliminary findings, CT chest should be considered along with CT abdomen and pelvis in symptomatic presurgical patients who require accurate and fast diagnosis in clinical settings where RT‐PCR is not readily available or has a long turnaround time (i.e. those who may need emergency laparotomy for an acute intra‐abdominal process). In asymptomatic presurgical patients (i.e. elective abdominal surgery patients), there is currently insufficient evidence to recommend routine preoperative CT chest. Further study is needed before definitive recommendations can be made.
Funding
This work did not receive any financial support or funding.
Conflicts of interest
BTH has been awarded education grants and speaker honoraria from W.L. Gore and Allergan. VA has received honoraria for speaking for Medtronic, Allergan, Intuitive, Acelity and W.L. Gore. All conflicts of interest are outside of the scope of the submitted work. JMS, SAA, EBD and SAE have no potential conflicts or disclosures.
Ethical approval
This work complies with all ethical standards.
References
- 1. Cao Z, Zhang Q, Lu X et al. Estimating the effective reproduction number of the 2019‐nCoV in China. medRxiv [Internet]. 2020. [cited 2020 Apr 16];2020.01.27.20018952. Available from: http://medrxiv.org/content/early/2020/01/29/2020.01.27.20018952.abstract [Google Scholar]
- 2. Liu Y, Gayle AA, Wilder‐Smith A, Rocklöv J. The reproductive number of COVID‐19 is higher compared to SARS coronavirus. J Travel Med 2020; 27: 1–4. [cited 2020 Apr 16];2020. Available from: https://academic.oup.com/jtm/article‐abstract/27/2/taaa021/5735319 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Onder G, Rezza G, Brusaferro S. Case‐fatality rate and characteristics of patients dying in relation to COVID‐19 in Italy. J Am Med Assoc 2020; 2019: 2019–20. [DOI] [PubMed] [Google Scholar]
- 4. Wu Z, McGoogan JM. Characteristics of and Important Lessons from the Coronavirus Disease 2019 (COVID‐19) Outbreak in China: Summary of a Report of 72314 Cases from the Chinese Center for Disease Control and Prevention. J Am Med Assoc 2020; 323: A1–2. Apr 7 [cited 2020 Apr 19]. [DOI] [PubMed] [Google Scholar]
- 5. Zhou F, Yu T, Du R et al. Clinical course and risk factors for mortality of adult inpatients with COVID‐19 in Wuhan, China: a retrospective cohort study. Lancet 2020; 395: 1054–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Jordan RE, Adab P, Cheng KK. Covid‐19: risk factors for severe disease and death. [cited 2020 Apr 19]; Available from: www.gov.uk/government/publications/covid‐19‐guidance‐on‐social‐distancing‐and‐for‐ [DOI] [PubMed] [Google Scholar]
- 7. Meng L, Qiu H, Wan L et al. Intubation and ventilation amid the COVID‐19 Outbreak. Anesthesiology 2020;132: 1317–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Lei S, Jiang F, Su W et al. Clinical characteristics and outcomes of patients undergoing surgeries during the incubation period of COVID‐19 infection. EClinicalMedicine [Internet]. 2020; 21: 100331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Aminian A, Safari S, Razeghian‐Jahromi A, Ghorbani M, Delaney CP. COVID‐19 Outbreak and Surgical Practice. Ann Surg 2020; 272: e27–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Nepogodiev D, Glasbey JC, Li E et al. Mortality and pulmonary complications in patients undergoing surgery with perioperative SARS‐CoV‐2 infection: an international cohort study. Lancet. 2020;396: 27–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Salathé M, Althaus CL, Neher R et al. COVID‐19 epidemic in Switzerland: on the importance of testing, contact tracing and isolation. Swiss Med Wkly 2020; 150: w20225. [DOI] [PubMed] [Google Scholar]
- 12. Wikramaratna P, Paton RS, Ghafari M, Lourenço J. Estimating false‐negative detection rate of SARS‐CoV‐2 by RT‐PCR. Available from: 10.1101/2020.04.05.20053355 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Xiao AT, Tong YX, Zhang S. False‐negative of RT‐PCR and prolonged nucleic acid conversion in COVID‐ 19: Rather than recurrence. J Med Virol [Internet] 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Li Y, Xia L. Role of chest CT in diagnosis and management. AJR Am J Roentgenol. 2020; 214: 1–7. [cited 2020 Apr 9] Available from: www.ajronline.org [DOI] [PubMed] [Google Scholar]
- 15. Bernheim A, Mei X, Huang M et al. Chest CT findings in coronavirus disease‐19 (COVID‐19): relationship to duration of infection. Radiology 2020; 295: 200463 [cited 2020 Apr 26]. Available from: http://pubs.rsna.org/doi/10.1148/radiol.2020200463 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Zhao W, Zhong Z, Xie X, Yu Q, Liu J. Relation between chest CT findings and clinical conditions of coronavirus disease (covid‐19) pneumonia. A multicenter study. Am J Roentgenol. 2020; 214: 1072–7. [DOI] [PubMed] [Google Scholar]
- 17. Pan F, Ye T, Sun P et al. Time course of lung changes on chest CT during recovery from 2019 novel Coronavirus (COVID‐19) Pneumonia. Radiology. 2020; 295: 715–21 [cited 2020 Apr 26]; 200370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Moher D, Shamseer L, Clarke M et al. Preferred reporting items for systematic review and meta‐analysis protocols (PRISMA‐P) 2015 statement. Rev Esp Nutr Humana y Diet. 2016; 20: 148–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Oxman AD. Grading quality of evidence and strength of recommendations. BMJ BMJ Publishing Group 2004; 328: 1490–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. QUADAS‐2, Bristol Medical School : Population Health Sciences University of Bristol [Internet]. [cited 2020 Jun 7]. Available from: https://www.bristol.ac.uk/population‐health‐sciences/projects/quadas/quadas‐2/ [Google Scholar]
- 21. He J‐L, Luo L, Luo Z‐D et al. Diagnostic performance between CT and initial real‐time RT‐PCR for clinically suspected 2019 coronavirus disease (COVID‐19) patients outside Wuhan, China. Respir Med 2020; 168: 105980. Apr 21 [cited 2020 Apr 28] Available from: http://www.sciencedirect.com/science/article/pii/S0954611120301207 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Li R, Tian J, Yang F et al. Clinical characteristics of 225 patients with COVID‐19 in a tertiary Hospital near Wuhan, China. J Clin Virol 2020; 1: 127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Miao C, Jin M, Miao L et al. Early chest computed tomography to diagnose COVID‐19 from suspected patients: A multicenter retrospective study. Am J Emerg Med [Internet]. 2020. Apr [cited 2020 Apr 27]; Available from: https://linkinghub.elsevier.com/retrieve/pii/S0735675720302813 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Wang K, Kang S, Tian R, Zhang X, Wang Y. Imaging manifestations and diagnostic value of chest CT of coronavirus disease 2019 (COVID‐19) in the Xiaogan area. Clin Radiol 2020; 75: 341–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Chen D, Jiang X, Hong Y et al. Can chest CT features distinguish patients with negative from those with positive initial RT‐PCR results for coronavirus disease (COVID‐19)? AJR Am J Roentgenol 2020: 1–5. [DOI] [PubMed] [Google Scholar]
- 26. Ai T, Yang Z, Hou H et al. Correlation of chest CT and RT‐PCR testing in coronavirus disease 2019 (COVID‐19) in China: A report of 1014 cases. Radiology 2020; 296: E32–E40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Fang Y, Zhang H, Xie J et al. Sensitivity of Chest CT for COVID‐19: Comparison to RT‐PCR. Radiology 2020;296:E115–E117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Wen Z, Chi Y, Zhang L et al. Coronavirus disease 2019: initial detection on chest CT in a retrospective multicenter study of 103 Chinese Subjects. Radiology 2020; 2. Radiological Society of North America (RSNA); Apr [cited 2020 Apr 28]. Available from: http://pubs.rsna.org/doi/10.1148/ryct.2020200092 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Bai HX, Hsieh B, Xiong Z et al. Performance of radiologists in differentiating COVID‐19 from viral pneumonia on chest CT. Radiology 2020; 296: E46–E54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Himoto Y, Sakata A, Kirita M et al. Diagnostic performance of chest CT to differentiate COVID‐19 pneumonia in non‐high‐epidemic area in Japan. Jpn J Radiol. 2020; 38: 400–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Dangis A, Gieraerts C, De BY et al. Accuracy and reproducibility of low‐dose submillisievert chest CT for the diagnosis of COVID‐19. Radiol Cardiothorac Imaging [Internet]. 2020; 2: e200196. [cited 2020 Apr 28]. Available from: http://pubs.rsna.org/doi/10.1148/ryct.2020200196 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Fu L, Luo M. Clinical and CT imaging characteristics of COVID‐ 19 cases in Wenzhou city: A retrospective analysis. Research Square. 2020; 1–20. [Google Scholar]
- 33. Caruso D, Zerunian M, Polici M et al. Chest CT features of COVID‐19 in Rome, Italy. Radiology 2020: 201237 [cited 2020 Apr 26]. Available from: http://pubs.rsna.org/doi/10.1148/radiol.2020201237 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Gietema HA, Zelis N, Nobel JM et al. CT in relation to RT‐PCR in diagnosing COVID‐19 in the Netherlands: a prospective study. PLOS One. 2020;15: e0235844 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Inui S, Fujikawa A, Jitsu M et al. Chest CT findings in cases from The Cruise Ship “Diamond Princess” with Coronavirus Disease 2019 (COVID‐19). Radiol Cardiothorac Imaging 2020; 2: e200110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Wang Y, Tong J, Qin Y et al. Characterization of an asymptomatic cohort of SARS‐COV‐2 infected individuals outside of Wuhan, China. PubMed [Internet]. [cited 2020 Jun 4]. Available from: https://academic.oup.com/cid/advance‐article‐abstract/doi/10.1093/cid/ciaa629/5842166 [Google Scholar]
- 37. Shi H, Han X, Jiang N et al. Radiological findings from 81 patients with COVID‐19 pneumonia in Wuhan, China: a descriptive study. Lancet Infect Dis 2020; 20: 425–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Khatami F, Saatchi M, Zadeh SST, Aghamir ZS, Shabestari AN, Aghamir SMK. Chest CT and RT‐PCR comparison in diagnosis of COVID‐19: A meta‐analysis. SSRN Electron J. 2020. [cited 2020 Apr 27]; Available from: https://www.ssrn.com/abstract=3576759 [Google Scholar]
- 39. Kim H, Hong H, Yoon SH. Diagnostic performance of CT and reverse transcriptase‐polymerase chain reaction for coronavirus disease 2019: A meta‐analysis. Radiology 2020: 201343 [cited 2020 Apr 27]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Rubin GD, Ryerson CJ, Haramati LB et al. The role of chest imaging in patient management during the COVID‐19 pandemic: a multinational consensus statement from the Fleischner society. Chest 2020; 158: 106–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Heffernan DS, Evans HL, Huston JM et al. Surgical infection society guidance for operative and peri‐operative care of adult patients infected by the severe acute respiratory syndrome coronavirus‐2 (SARS‐CoV‐2). Surg Infect (Larchmt) 2020; 21: 301–8 [cited 2020 Apr 26] sur.2020.101. Available from: https://www.liebertpub.com/doi/10.1089/sur.2020.101 [DOI] [PubMed] [Google Scholar]
- 42. Kang Z, Li X, Zhou S. Recommendation of low‐dose CT in the detection and management of COVID‐2019. Eur Radiol. 2020; 30: 4356–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Judson SD, Munster VJ. Nosocomial transmission of emerging viruses via aerosol‐generating medical procedures. Viruses 2019; 11: 940. Available from: https://www.mdpi.com/1999‐4915/11/10/940 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Workman AD, Welling DB, Carter BS et al. Endonasal instrumentation and aerosolization risk in the era of COVID‐19: simulation, literature review, and proposed mitigation strategies. Int Forum Allergy Rhinol. 2020; 10: 798–805 Apr 3 [cited 2020 Apr 26]; Available from: http://doi.wiley.com/10.1002/alr.22577 [DOI] [PubMed] [Google Scholar]
- 45. The Royal College of Radiologists . Statement on use of CT chest to screen for COVID‐19 in pre‐operative patients [Internet]. [cited 2020 May 4]. Available from: https://www.rcr.ac.uk/college/coronavirus‐covid‐19‐what‐rcr‐doing/clinical‐information/statement‐use‐ct‐chest‐screen‐covid [Google Scholar]
- 46. Wang YXJ, Liu W‐H, Yang M, Chen W. The role of CT for Covid‐19 patient’s management remains poorly defined. Ann Transl Med 2020; 8: 145–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. FDA . What are the Radiation Risks from CT? [Internet]. [cited 2020 Apr 26]. Available from: https://www.fda.gov/radiation‐emitting‐products/medical‐x‐ray‐imaging/what‐are‐radiation‐risks‐ct [Google Scholar]
- 48. Chirurgisch onderzoek: SCOUT‐1 en SCOUT‐2 studie ‐ evaluatie opbrengst (1) en bepaling accuratesse (2) preoperatieve screening | NVvH | De Nederlandse Vereniging voor Heelkunde [Internet]. [cited 2020 May 4]. Available from: https://heelkunde.nl/nieuws/chirurgisch‐onderzoek‐scout‐1‐en‐scout‐2‐studie‐evaluatie‐opbrengst‐1‐en‐bepaling‐accuratesse [Google Scholar]


