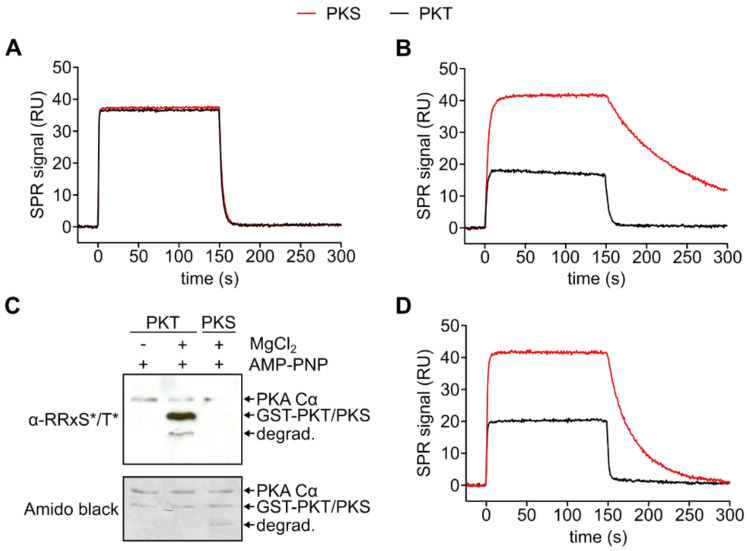
Figure 3.
SPR analysis of the formation of the Michaelis complex. (A) Comparison of the interaction between PKA Cα (900 nM) and GST-PKS (red) and GST-PKT (black), respectively, in the absence of metal ions and nucleotides (Figure S4). Both binding kinetics are similar. (B) Comparison of the interaction of PKA Cα (100 nM) with GST-PKS (red) and GST-PKT (black), respectively, in the presence of 0.2 mM AMP-PNP and 1 mM MgCl2 (Figure S5). The Michaelis complex with PKS is stabilized while in the case of PKT, the complex dissociates faster. (C) Western blot with a phospho-PKA substrate antibody (α-RRXS*/T*). Phosphorylation of GST-PKT by PKA Cα can be detected after 5 min incubation at 25 °C in the presence of 0.2 mM AMP-PNP and 1 mM MgCl2. (D) Interaction of 300 nM PKA Cα with GST-PKS (red) and GST-PKT (black) in the presence of 0.2 mM AMP-PCP and 1 mM MnCl2 (Figure S6). The complex with PKS can be stabilized with Mn2+ ions, while the PKT Michaelis complex dissociates fast.