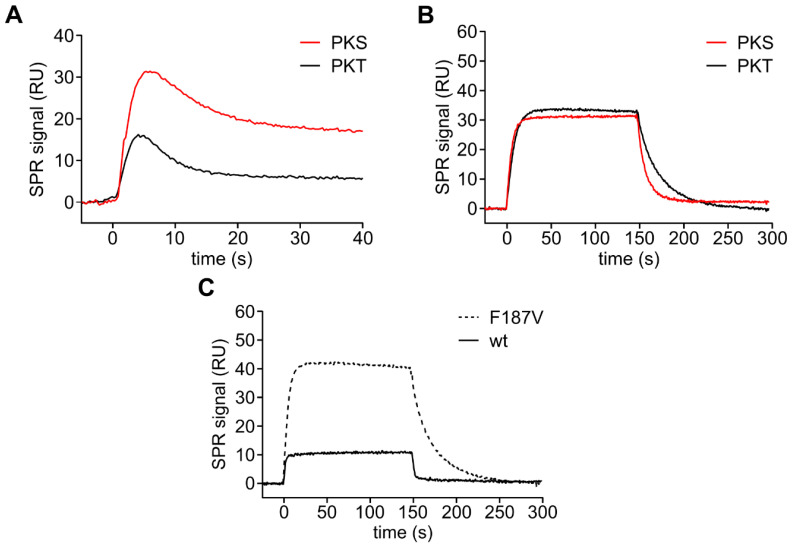
Figure 4.
PKA Cα F187V has a higher affinity for the threonine substrate PKT. (A) On-chip phosphorylation of GST-PKS (red) and GST-PKT (black) with 100 nM PKA Cα F187V (Figure S9B,C). PKA Cα F187V phosphorylates GST-PKT faster than GST-PKS. (B) SPR analyses of the interaction between 50 nM PKA Cα F187V and GST-PKS (red) and GST-PKT (black), respectively, in the presence of 0.2 mM AMP-PCP and 1 mM MnCl2 (Figure S9D,E). The mutant shows a slightly slower dissociation for the threonine substrate PKT. (C) Comparison of the binding curves of PKA Cα wt (solid line) and F187V (dashed line; 100 nM each) demonstrates that the mutant has a higher affinity for the threonine substrate PKT as the wt. See Table 4 for the determined rate constants and KD values.