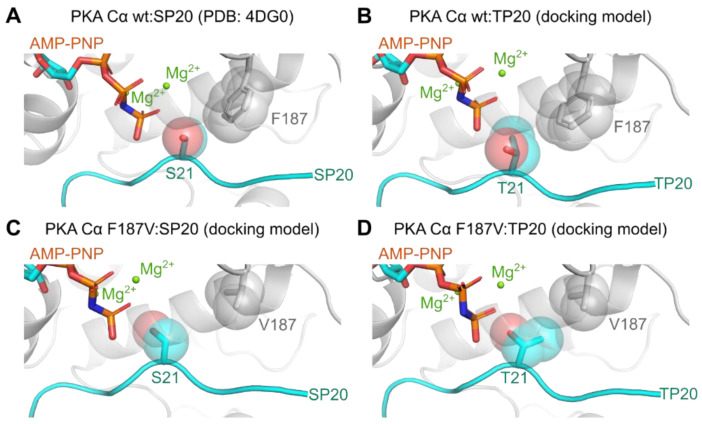
Figure 5.
The Ser/Thr specificity of PKA is determined by the DFG+1 motif. (A) The binding pocket of PKA Cα in complex with AMP-PNP, Mg2+, and SP20 (PDB code: 4DG0) [25]. The DFG+1 phenylalanine (F187) occupies a large space directly adjacent to the P0 site, coordinating the SP20 serine at this position. (B–D) Model structures derived from docking simulations performed with YASARA (v.18.12.27) [41,52]. (B) The hydroxyl group of the threonine at the P0 site (T21 in TP20) points away from the γ-phosphate due to the additional methyl group, which could hinder the phosphoryl transfer. (C) In the case of the F187V mutation, the P0 serine can rotate more freely due to the smaller branched valine at the DFG+1 position. (D) The valine could allow a favorable orientation of the threonine at the P0 site for phosphoryl transfer. All structure images were generated using the PyMOL Molecular Graphics System (Version 2.2.2; Schrödinger, LLC, New York, NY, USA).