Fig. 2.
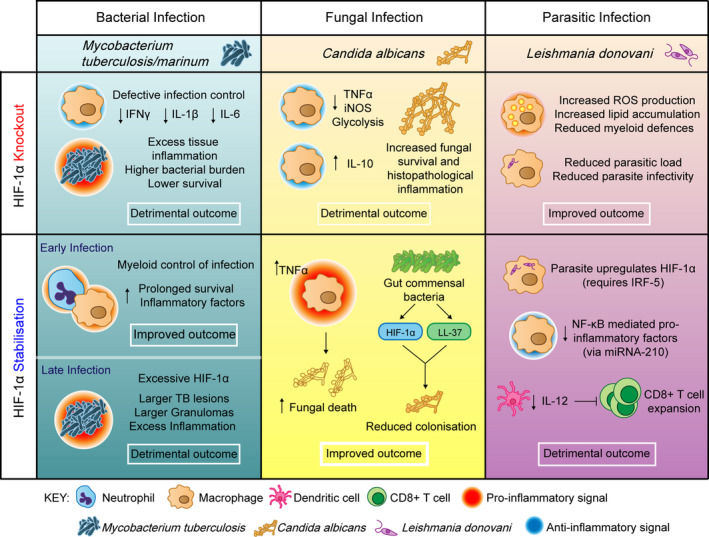
The contribution of HIF‐1α to infection outcomes. Figure showing the effects of HIF manipulation on bacterial, fungal and parasitic infection outcomes. Generally, HIF‐1α stabilisation is beneficial in the context of bacterial infections, for example Mycobacterium tuberculosis/marinum (blue column); however, the level of HIF‐1α must be carefully balanced, with too little or too much resulting in excess inflammation and detrimental infection outcome. HIF‐1α knockouts have decreases in IFNγ [77], IL‐1β [51] and IL‐6 [51] leading to excess tissue damage and lower survival [46]. Early HIF‐1α stabilisation increases inflammatory factors leading to myeloid control of infection [48, 49, 50], while excessive HIF‐1α can lead to prolonged inflammation and larger TB lesions in later stage disease [51]. In the context of the fungal infection Candida albicans (yellow column), HIF‐1α knockout decreases pro‐inflammatory factors while increasing anti‐inflammatory IL‐10 leading to increased fungal survival [55]. HIF‐1α stabilisation improves infection outcome, re‐arming the host inflammatory response leading to increased fungal death [55, 58]. In the parasitic infection Leishmania donovani (purple column), reducing HIF‐1α improves infection outcome, as the parasite itself upregulates host HIF‐1α, and stabilised HIF‐1α prevents CD8 + T‐cell expansion, exacerbating the infection further [62, 63, 64, 78]. Each infection context is distinct, requiring a tailored and controlled HIF‐α response that is not uniform across infections.
