Abstract
To investigate the factors associated with the duration of severe acute respiratory syndrome coronavirus 2 RNA shedding in patients with coronavirus disease 2019 (COVID‐19). A retrospective cohort of COVID‐19 patients admitted to a designated hospital in Beijing was analyzed to study the factors affecting the duration of viral shedding. The median duration of viral shedding was 11 days (IQR, 8‐14.3 days) as measured from illness onset. Univariate regression analysis showed that disease severity, corticosteroid therapy, fever (temperature>38.5°C), and time from onset to hospitalization were associated with prolonged duration of viral shedding (P < .05). Multivariate regression analysis showed that fever (temperature>38.5°C) (OR, 5.1, 95%CI: 1.5‐18.1), corticosteroid therapy (OR, 6.3, 95%CI: 1.5‐27.8), and time from onset to hospitalization (OR, 1.8, 95%CI: 1.19‐2.7) were associated with increased odds of prolonged duration of viral shedding. Corticosteroid treatment, fever (temperature>38.5°C), and longer time from onset to hospitalization were associated with prolonged viral shedding in COVID‐19 patients.
Keywords: coronavirus, COVID‐19, duration of viral shedding
Highlights
Corticosteroid treatment was associated with prolonged viral shedding in COVID‐19 patients
1. INTRODUCTION
Coronavirus disease 2019 (COVID‐19) caused by severe acute respiratory syndrome coronavirus 2 (SARS‐CoV‐2) has attracted worldwide attention since January 2020. According to the World Health Organization, as of 16 June 2020, almost 8 million people throughout the world have been infected with COVID‐19. Due to the global outbreak of coronavirus infection, a comprehensive understanding of the natural course of infection and the potential period of infection is very important in preventing the further spread of this novel coronavirus. Studies on the epidemiology, clinical characteristics and risk factors for mortality of COVID‐19 have been carried out, of which, results on the duration of viral shedding (DVS) vary, and the factors affecting the time of persistent positive nucleic acid have not been reported. Here, we describe the results of our analysis on the DVS in a retrospective cohort of COVID‐19 patients in Beijing, China. We aim to determine the risk factors affecting the period of virus positivity, especially the effect of controversial corticosteroid therapy on the viral shedding time, to provide evidence‐based medicine for the treatment of COVID‐19.
2. PATIENTS AND METHODS
2.1. Study design and participants
This retrospective cohort study included 101 consecutive patients with COVID‐19 who were hospitalized in Beijing YouAn Hospital from 21 January to 15 March 2020. The diagnosis of COVID‐19 was required to fulfill the criteria of the WHO interim guidance. 1
2.2. Ethical approval
The study was approved by the Ethics Committee of Beijing YouAn Hospital, Capital Medical University‐2020‐31, and the requirement for informed consent was waived by the Ethics Committee.
2.3. Data collection
Patient data concerning the recent history of epidemiological exposure, clinical symptoms or signs, treatment regimen, and all laboratory results during hospitalization were collected using electronic medical records. Laboratory indicators included routine blood test, blood chemical analysis, coagulation index, C‐reactive protein, procalcitonin, and creatine kinase. Patients were followed up for 2 weeks, and the results of detectable nucleic acid during hospitalization and after 2 weeks of follow‐up were obtained.
2.4. Definitions
The DVS is defined as the time from illness onset to successive negative detection of SARS‐CoV‐2 RNA. 2 , 3 The end of viral shedding was determined by at least two consecutive negative throat‐swab samples by real‐time polymerase chain reaction (RT‐PCR) analysis and if all patients were still negative after 2 weeks of follow‐up.
Disease severity is defined as follows: (a) general: only mild symptoms, with or without fever, and imaging shows pneumonia. (b) severe: if any of the following criteria are met: (a) respiratory distress, respiratory rate more than equal to 30 beats/minute; (b) in the resting state, means oxygen saturation less and equal to 93%; (c) arterial blood oxygen partial pressure/oxygen concentration less than equal to 300 mm Hg (1 mm Hg = 0.133kPa). Chest imaging showed that the lesions progressed more than 50% within 24 to 48 hours, and the patients were managed according to general cases. (a) critical, if one of the following conditions was present: (a) respiratory failure requiring mechanical ventilation; (b) the presence of shock; (c) ICU admission is required for multiple organ failure.
2.5. Statistical analysis
The patients were divided into two groups according to the DVS: patients with a long DVS (>median days) and patients with a short DVS (≤median days), and we chose the variables with significant differences between the two groups to conduct a multivariate logistic regression model. As there were too few dependent variables in our study, to avoid overfitting the model, according to previous research results and clinical constraints, five variables that were most likely to affect DVS were selected for multivariate analysis. A two‐sided α of less than ·05 was considered statistically significant. All statistical analyses were performed using the Statistical Package for Social Sciences ver. 23.0 (SPSS, Chicago, IL).
3. RESULTS
3.1. Demographic and clinical characteristics of the patients in this study
A total of 101 patients were diagnosed with COVID‐19 during the study period. Of these, three patients whose viral nucleic acid was still detected before death during hospitalization were excluded. Nine patients without a definite time of virus shedding were also excluded. To ensure the accuracy of the time of onset, patients under 18 years old (n = 6) and patients whose time from onset to hospitalization was more than 7 days (n = 17) were excluded. The remaining 66 patients were included in the data analysis (Figure 1). The median age of these 66 patients was 47.5 years (IQR, 36‐63.5 years) and 39.4% of the enrolled patients were female (Table 1).
Figure 1.
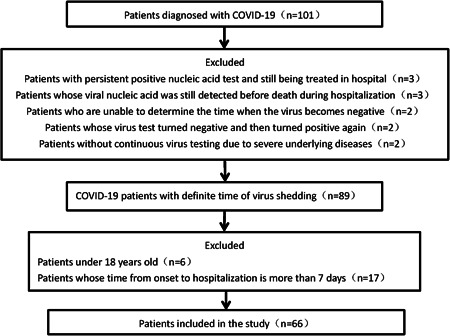
Patient screening and enrollment process. COVID‐19, coronavirus disease 2019
Table 1.
Clinical characteristics of COVID‐19 patients with different duration of viral shedding
| Total (n = 66) | DVS ≤ 11 d (n = 35) | DVS>11 d (n = 31) | P | |
|---|---|---|---|---|
| Demographics and clinical characteristics | ||||
| Age, y | 47.5 (36‐63.5) | 46 (31‐60) | 59 (39‐67) | .042 |
| Female | 26 (39.39%) | 10 (28.6%) | 16 (51.6%) | .056 |
| Smoker | 4 (6.1%) | 2 (5.7%) | 2 (6.5%) | .900 |
| Comorbidity | ||||
| Hypertension | 19 (28.79) | 7 (20%) | 12 (38.7%) | .094 |
| Diabetes | 6 (9.09%) | 3 (8.6%) | 3 (9.7%) | .876 |
| Cerebrovascular diseases | 8 (12.12%) | 4 (11.4%) | 4 (12.9%) | .855 |
| Chronic obstructive lung disease | 2 (3.03%) | 0 (0%) | 2 (6.5%) | .217 |
| Chronic kidney disease | 1 (1.51%) | 1 (2.9%) | 0 (0%) | 1.000 |
| Chronic liver disease | 6 (9.09%) | 2 (5.7%) | 4 (12.9%) | .559 |
| Carcinoma | 3 (4.55%) | 1 (2.9%) | 2 (6.5%) | .914 |
| T max (temperature>38.5°C) | 28 (42.42%) | 9 (25.7%) | 19 (61.3%) | .004 |
| Disease severity | .004 | |||
| General | 46 (69.69%) | 30 (85.7%) | 16 (51.6%) | |
| Severe | 11 (16.67%) | 4 (11.4%) | 7 (22.6%) | |
| Critical | 9 (13.63%) | 1 (2.9%) | 8 (25.8%) | |
| Time from onset to hospitalization, d | 4 (3‐5) | 4 (2‐5) | 5 (4‐6) | .008 |
| Laboratory findings | ||||
| White blood cell count, ×109/L | 4.11 (3.445‐5.66) | 3.95 (3.455.42) | 4.26 (3.43‐6.49) | .379 |
| Lymphocyte count, ×109/L | 1.1 (0.76‐1.5) | 1.2 (0.77‐1.49) | 0.99 (0.75‐1.53) | .181 |
| Hemoglobin, g/L | 137.5 (124.75‐144.25) | 133 (123‐142) | 141 (133‐152) | .041 |
| Anemia | 7 (10.61%) | 4 (11.4%) | 3 (9.7%) | .817 |
| Platelet count, ×109/L | 178 (148.75‐219.5) | 190 (148‐257) | 168 (151‐209) | .107 |
| Prothrombin time, s | 12.6 (11.9‐13.1) | 12.4 (11.9‐13) | 12.7 (12‐13.2) | .541 |
| ALT, U/L | 28 (21‐46.25) | 28 (19‐46) | 28 (23‐52) | .252 |
| AST, U/L | 29.5 (20.75‐42) | 29 (19‐41) | 30 (23‐50) | .289 |
| Albumin, g/L | 37.15 (33.675‐39.8) | 37.4 (34‐39.3) | 36.7 (32‐41) | .959 |
| Creatine kinase, U/L | 70 (46‐116) | 60.5 (45.75‐98.75) | 87 (46‐142) | .219 |
| Creatinine, μmol/L | 63.5 (54.5‐77.25) | 61 (53‐74) | 66 (58‐78) | .452 |
| C‐reactive protein, mg/L | 13.6 (2.6‐28.65) | 14 (2.3‐26.3) | 13.5 (3.2‐51.1) | .396 |
| Procalcitonin, ng/mL | 0.11 (0.1‐0.14) | 0.12 (0.1‐0.14) | 0.11 (0.1‐0.14) | .916 |
| Treatments | ||||
| Antibiotics | 7 (10.61%) | 1 (2.9%) | 6 (19.4%) | .076 |
| Antiviral treatment | 40 (60.61%) | 23 (65.7%) | 17 (54.8%) | .367 |
| TCM treatment | 58 (87.88%) | 30 (88.2%) | 28 (93.3%) | .788 |
| Corticosteroids | 17 (25.76%) | 4 (11.4%) | 13 (41.9%) | .005 |
| Oxygen support therapy | 62 (93.94%) | 33 (94.3%) | 29 (93.5%) | .900 |
| Noninvasive mechanical ventilation | 3 (4.55%) | 0 (0%) | 3 (9.7%) | .196 |
| Invasive mechanical ventilation | 3 (4.55%) | 0 (0%) | 3 (9.7%) | .196 |
| ECMO | 2(3.03%) | 0 (0%) | 2 (6.5%) | .420 |
| Outcomes | ||||
| ARDS | 7 (10.61) | 2 (5.7%) | 5 (16.1%) | .332 |
| Septic shock | 4 (6.06%) | 0 (0%) | 4 (12.9%) | .094 |
| Acute cardiac injury | 4 (6.06%) | 0 (0%) | 4 (12.9%) | .094 |
| Acute kidney injury | 3 (4.55%) | 0 (0%) | 3 (9.7%) | .196 |
| Acute liver injury | 23 (34.85%) | 10 (28.6%) | 13 (41.9%) | .255 |
| Secondary infection | 7 (10.61%) | 1 (2.9%) | 6 (19.4%) | .076 |
| ICU admission | 5 (7.57) | 0 (0%) | 5 (16.1%) | .045 |
| Death | 2 (3.03%) | 0 (0%) | 2 (6.5%) | .420 |
| Hospital length of stay, d | 15 (10.25‐18) | 13 (10‐16) | 17 (12.5‐18) | .040 |
Abbreviations: ALT, alanine aminotransferase; ARDS, adult respiratory distress syndrome; AST, aspartate transaminase; COVID‐19, coronavirus disease 2019; DVS, duration of viral shedding; ECMO, extracorporeal membrane oxygenation; ICU, intensive care unit.
3.2. Duration of viral shedding
The median DVS was 11 days (IQR, 8‐14.3 days), and the DVS in 35 patients was more than 11 days. The median age of the patients in the short DVS group was 46 years, which was significantly lower than that in the long DVS group (59 years, P = .042); 25.7% of patients in the short DVS group had a fever, while 61.3% of patients in the long DVS group had a fever, the difference between the two groups was statistically significant (P = .004). The number of general, severe, and critical cases in the short DVS group was 30, 4, and 1, respectively, and was 16, 7, and 8, respectively, in long DVS group (P = .004) (Table 1). The DVS in the general, severe and critical patient groups was 9.5 days (IQR, 8‐13), 12 days (IQR, 9‐19), and 17 days (IQR, 13.5‐18.5), respectively, with significant differences between the groups (P = .004). The time from onset to hospitalization in the short DVS group was shorter than that in the long DVS group (4 vs 5 days, P = .008). The median time from illness onset to discharge was 13.0 days (IQR, 10.0–16.0) in the short DVS group, and was 17.0 days (IQR, 12.5–18.0) in the long DVS group (P = .040). The proportion of patients treated with corticosteroid therapy in the long DVS group was significantly higher than that in the short DVS group (41.9% vs 11.4%; P = .005). Similarly, the number of patients admitted to the ICU in the long DVS group was also significantly higher than that in the short DVS group (16.1% vs 0%; P = .045).
3.3. Factors associated with DVS
Univariate regression analysis showed that disease severity, corticosteroid therapy, fever, and the time from onset to hospitalization were associated with a long DVS (Table 2). When the above variables were included in the multivariate logistic regression model, it was found that fever (temperature >38.5°C) (OR, 5.1, 95%CI: 1.5‐18.1), corticosteroid therapy (OR, 6.3, 95%CI: 1.5‐27.8), and the time from onset to hospitalization (OR, 1.8, 95%CI: 1.19‐2.7) were associated with increased odds of a long DVS (P < .05). We further analyzed the relationship between the maximum body temperature and DVS and found that DVS was gradually prolonged with increased maximum body temperature (Figure 2). Maximum body temperature was divided into three groups: less than 37.3°C, 37.3 to 38.5°C, and more than equal to 38.5°C, and the DVS in each group was shown to be 9 days (IQR, 7‐11), 11 days (IQR, 7‐13), and 12.5 days (IQR, 9‐17), respectively, (P = .046).
Table 2.
Risk factors associated with prolonged duration of viral shedding in coronavirus disease 2019 patients
| Univariate | Multivariate | |||
|---|---|---|---|---|
| Variables | OR (95% CI) | P | OR (95% CI) | P |
| Age* | 1.03 (0.99‐1.06) | .054 | ||
| Disease severity | ||||
| General | ref | |||
| Severe | 3.3 (0.8‐12.9) | .089 | ||
| Critical | 14.9 (1.7‐130.8) | .014 | ||
| Corticosteroid | ||||
| No | ref | ref | ||
| Yes | 5.6 (1.6‐19.8) | .007 | 6.3 (1.5‐27.8) | .014 |
| T max (temperature) | ||||
| ≤38.5°C | ref | ref | ||
| >38.5°C | 4.6 (1.6‐13.0) | .004 | 5.1 (1.5‐18.1) | .011 |
| Time from onset to hospitalization*, d | 1.5 (1.1‐2.0) | .012 | 1.8 (1.19‐2.7) | .005 |
continuous variable
Figure 2.
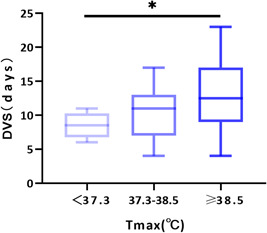
Relationship between maximum body temperature and DVS. The Kruskal–Wallis test was used to compare the groups. DVS, duration of viral shedding
Patients who were treated with corticosteroids were found to have a 6.3‐fold increased risk of a long DVS, independent of their age, maximum body temperature, time from onset to hospitalization, and disease severity (Table 2). The corticosteroid therapy effect may be dose‐dependent and may vary according to the time of initiation; therefore, subgroup analysis of the following stratified groups was carried out: patients who received high‐dose corticosteroid therapy (total dose of >300 mg methylprednisolone) and patients who received low‐dose corticosteroid therapy (total dose ≤300 mg methylprednisolone). However, a statistically significant difference in the DVS between the two dose groups was not found (15.5 vs 16 days, P > .05) (Figure 3). The detectability (percentage of patients) of SARS‐CoV‐2 RNA in patients treated with and without corticosteroids across time is depicted in Figure 1. The median DVS in the corticosteroid treated and the untreated group was 16 days (IQR, 11.5‐18.5 days) and 10 days (IQR, 8‐13 days), respectively, (P = .000). The cumulative proportion of patients with the undetectable virus at 6 to 8, 9 to 11, 12 to 14, 15 to 17 and 18 to 20 days from illness onset in the untreated group were 29.1%, 59.6%, 86.0%, 95.9%, and 98%, respectively, significantly higher than those in the corticosteroid treated group (Figure 4).
Figure 3.
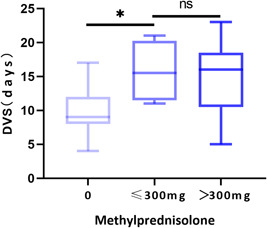
Relationship between the dose of methylprednisolone and DVS. The Kruskal–Wallis test was used to compare the groups. *P < .05, DVS, duration of viral shedding; ns, no statistical significance
Figure 4.
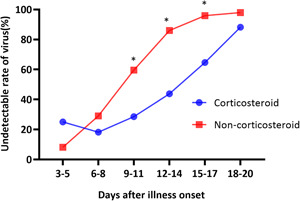
Rate of undetectable SARS‐CoV‐2 RNA in patients during the first 20 days of illness. The Chi square test was used to compare the two groups at each time point. *P < .05. SARS‐CoV‐2, severe acute respiratory syndrome coronavirus 2
4. DISCUSSION
This retrospective study describes the DVS in COVID‐19 patients whose nucleic acid test was still negative after 2 weeks follow‐up, and the median DVS in these patients was 11 days (IQR, 8‐14.3)(Table 1). In this study, the risk factors associated with prolonged DVS in adults who were hospitalized within 7 days of illness onset were investigated. It was found that severe/critical disease, corticosteroid therapy, fever, and shorter time from onset to hospitalization were associated with higher odds of a long DVS (Table 2). Multivariate regression analysis showed that fever, corticosteroid therapy, and time from onset to hospitalization were independent risk factors for a long DVS in COVID‐19 patients. Further analysis showed that the DVS was gradually prolonged with increased maximum body temperature (Figure 2).
Recently, a retrospective study of COVID‐19 patients in Wuhan 4 showed that the median DVS was 20.0 days (IQR, 17.0‐24.0) in survivors, which was significantly longer than that in our patients, the reason for this result may be due to the heterogeneity of patients in the two studies. Outside Wuhan, the results of a study 5 from Beijing showed that the viral load in throat swabs and sputum of two COVID‐19 patients peaked about 6 days after the onset of symptoms, and the virus could not be detected on the 9th and 12th day after onset, respectively. The results of this study are consistent with the DVS in patients included in our study. A retrospective study 6 from Shanghai, China showed that the median time from the onset of symptoms to the first negative RT‐PCR results for oropharyngeal swabs in convalescing patients was 9.5 (6.0‐11.0) days. Another retrospective study 7 from Guangdong, China showed that the median DVS in 84 COVID‐19 patients was 15 days, and the patients included in the study were not required to be hospitalized within 1 week after onset and followed up for 2 weeks. Thus, it can be seen that the DVS in COVID‐19 patients in existing research results is quite different, and is considered to be related to the heterogeneity of the study population, and different medical conditions in the study area.
At present, COVID‐19 is spreading rapidly in many countries around the world. It is of great significance to understand the period of infection to control the pandemic. The present study found that the factors influencing the time of nucleic acid shedding were age, disease severity, the time from onset to hospitalization, maximum body temperature, and corticosteroid therapy. A recent study 7 on factors associated with prolonged viral RNA shedding in patients with COVID‐19 showed that invasive mechanical ventilation was an independent risk factor for prolonged SARS‐CoV‐2 RNA shedding, which indirectly reflects the severity of illness and could affect nucleic acid clearance, which is consistent with our study findings. However, multivariate regression analysis did not identify disease severity as a factor influencing the DVS. This result may be attributed to the fact that the patients in this study had mainly mild disease and the sample size was small.
The results of this study suggested that the higher the COVID‐19 patient's temperature, the longer the patient showed persistent positive nucleic acid. This result is consistent with a retrospective study 8 in children with COVID 19, which also showed that prolonged DVS in children with COVID‐19 was associated with fever. We consider that the patient's temperature is related to the inflammatory response caused by the host immune response. However, cytokine levels in our patients were not determined; thus, the cause of prolonged viral clearance associated with high fever is unclear.
The administration of corticosteroids in the treatment of viral pneumonia is controversial. Although, some western scholars 9 have suggested that there are no clinical data to indicate that a net benefit is derived from corticosteroids in the treatment of respiratory infection due to respiratory syncytial virus, influenza, SARS‐CoV, or Middle East respiratory syndrome coronavirus (MERS‐CoV). However, Current studies 10 , 11 have demonstrated that appropriate use of low‐dose corticosteroids may have survival advantages in critically ill patients with COVID‐19. On the other hand, many studies 12 , 13 , 14 , 15 have shown that corticosteroid therapy can prolong the shedding time of SARS, MERS, and influenza virus, but there are few studies on COVID‐19. In the present study, it was shown that corticosteroid therapy can delay the shedding time of SARS‐CoV‐2 RNA, which was similar to another recent study on the shedding time of nucleic acid in patients with COVID‐19. 6 In terms of the dose of corticosteroid therapy, we did not find a difference in the nucleic acid shedding time between the high dose and low dose groups (Figure 3). A retrospective study 13 adjusted for baseline and time‐varying confounders also found that early initiation of corticosteroid therapy, but not the dose, was associated with delayed MERS‐CoV RNA clearance. But, in patients infected with H7N9 avian influenza, the detectable time of influenza virus nucleic acid in respiratory tract samples was prolonged when the daily dose of methylprednisolone was more than 150 mg. 14 Thus, the association between the dose and time of initiation of corticosteroid therapy and the DVS in COVID‐19 patients requires further study. Also, the DVS was strongly related to the host immune status in immunocompromised patients with influenza A. In immunocompromised patients, the shedding time of influenza A virus was prolonged, 16 and it was speculated that corticosteroid therapy will affect the immune status of patients, and then affect the virus shedding time.
Our study has some limitations. First, there may be a certain bias in the results of this study as the sample size was small and the number of critically ill patients was low. Second, we only analyzed nucleic acid in throat swabs, and not in blood, sputum, feces, or anal swabs. However, it has been reported 17 , 18 that the duration of nucleic acid in sputum or feces is longer than that in throat swabs. Third, there are only qualitative results of nucleic acid detection in this study, but the persistent positivity of SARS‐CoV‐2 RNA does not necessarily indicate persistent shedding of live virus.
5. CONCLUSION
This study demonstrated that corticosteroid treatment, fever, and longer time from onset to hospitalization were associated with prolonged duration of viral shedding in COVID‐19 patients. Therefore, patients with COVID‐19 should be hospitalized promptly and the indications for corticosteroid therapy strictly controlled.
CONFLICT OF INTERESTS
The authors declare that there are no conflict of interests.
AUTHOR CONTRIBUTIONS
T‐ZL was responsible for the clinical data collection, data analysis and drafting of the manuscript. Z‐HC contributed to the data analysis. M‐TC, L‐YZ, HX, J‐YZ, C‐HM, Y‐L, L‐JG, and Z‐HD contributed to the data collection. YC, D‐LM, and L‐CL contributed to the study design and manuscript revision.
Li T‐Z, Cao Z‐H, Chen Y, et al. Duration of SARS‐CoV‐2 RNA shedding and factors associated with prolonged viral shedding in patients with COVID‐19. J Med Virol. 2021;93:506–512. 10.1002/jmv.26280
REFERENCES
- 1. Organization WH Clinical management of severe acute respiratory infection when novel coronavirus (2019‐nCoV) infection is suspected: interim guidance. 2020.
- 2. Qi L, Yang Y, Jiang D, et al. Factors associated with duration of viral shedding in adults with COVID‐19 outside of Wuhan, China: a retrospective cohort study. Int J Infect Dis. 2020;96:531‐537. 10.1016/j.ijid.2020.05.045 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Hao S, Lian J, Lu Y, et al. Decreased B cells on admission was associated with prolonged viral RNA shedding from respiratory tract in coronavirus disease 2019: a case control study. J Infect Dis. 2020;222:367‐371. 10.1093/infdis/jiaa311 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Zhou F, Yu T, Du R, et al. Clinical course and risk factors for mortality of adult inpatients with COVID‐19 in Wuhan, China: a retrospective cohort study. Lancet (London, England). 2020;395:1054‐1062. 10.1016/S0140-6736(20)30566-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Pan Y, Zhang D, Yang P, Poon LLM, Wang Q. Viral load of SARS‐CoV‐2 in clinical samples. Lancet Infect Dis. 2020;20:411‐412. 10.1016/S1473-3099(20)30113-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Ling Y, Xu SB, Lin YX, et al. Persistence and clearance of viral RNA in 2019 novel coronavirus disease rehabilitation patients. Chin Med J. 2020;133:1039‐1043. 10.1097/CM9.0000000000000774 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Xu K, Chen Y, Yuan J, et al. Factors associated with prolonged viral RNA shedding in patients with COVID‐19 [published online ahead of print April 9, 2020]. Clin Infect Dis. 2020. 10.1093/cid/ciaa351 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Lu Y, Li Y, Deng W, et al. Symptomatic infection is associated with prolonged duration of viral shedding in mild coronavirus disease 2019: a retrospective study of 110 children in Wuhan. Pediatr Infect Dis J. 2020;39(7):e95‐e99. 10.1097/INF.0000000000002729 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Russell CD, Millar JE, Baillie JK. Clinical evidence does not support corticosteroid treatment for 2019‐nCoV lung injury. Lancet (London, England). 2020;395(10223):473‐475. 10.1016/S0140-6736(20)30317-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Zhou W, Liu Y, Tian D, et al. Potential benefits of precise corticosteroids therapy for severe 2019‐nCoV pneumonia. Signal Transduct Target Ther. 2020;5:18. 10.1038/s41392-020-0127-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Wu C, Chen X, Cai Y, et al. Risk factors associated with acute respiratory distress syndrome and death in patients with coronavirus disease 2019 pneumonia in Wuhan, China. JAMA Intern Med. 2020;180(7):934‐943. 10.1001/jamainternmed.2020.0994 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Lee N, Allen Chan KC, Hui DS, et al. Effects of early corticosteroid treatment on plasma SARS‐associated Coronavirus RNA concentrations in adult patients. J Clin Virol. 2004;31(4):304‐309. 10.1016/j.jcv.2004.07.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Arabi YM, Mandourah Y, Al‐Hameed F, et al. Corticosteroid therapy for critically ill patients with Middle East respiratory syndrome. Am J Respir Crit Care Med. 2018;197(6):757‐767. 10.1164/rccm.201706-1172OC [DOI] [PubMed] [Google Scholar]
- 14. Cao B, Gao H, Zhou B, et al. Adjuvant corticosteroid treatment in adults with influenza A (H7N9) viral pneumonia. Crit Care Med. 2016;44(6):e318‐e328. 10.1097/CCM.0000000000001616 [DOI] [PubMed] [Google Scholar]
- 15. Auyeung TW, Lee JS, Lai WK, et al. The use of corticosteroid as treatment in SARS was associated with adverse outcomes: a retrospective cohort study. J Infect. 2005;51(2):98‐102. 10.1016/j.jinf.2004.09.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Weinstock DM, Gubareva LV, Zuccotti G. Prolonged shedding of multidrug‐resistant influenza A virus in an immunocompromised patient. N Engl J Med. 2003;348(9):867‐868. 10.1056/NEJM200302273480923 [DOI] [PubMed] [Google Scholar]
- 17. Zhang W, Du RH, Li B, et al. Molecular and serological investigation of 2019‐nCoV infected patients: implication of multiple shedding routes. Emerg Microbes Infect. 2020;9(1):386‐389. 10.1080/22221751.2020.1729071 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Wu Y, Guo C, Tang L, et al. Prolonged presence of SARS‐CoV‐2 viral RNA in faecal samples. Lancet Gastroenterol Hepatol. 2020;5(5):434‐435. 10.1016/S2468-1253(20)30083-2 [DOI] [PMC free article] [PubMed] [Google Scholar]


