Figure 2.
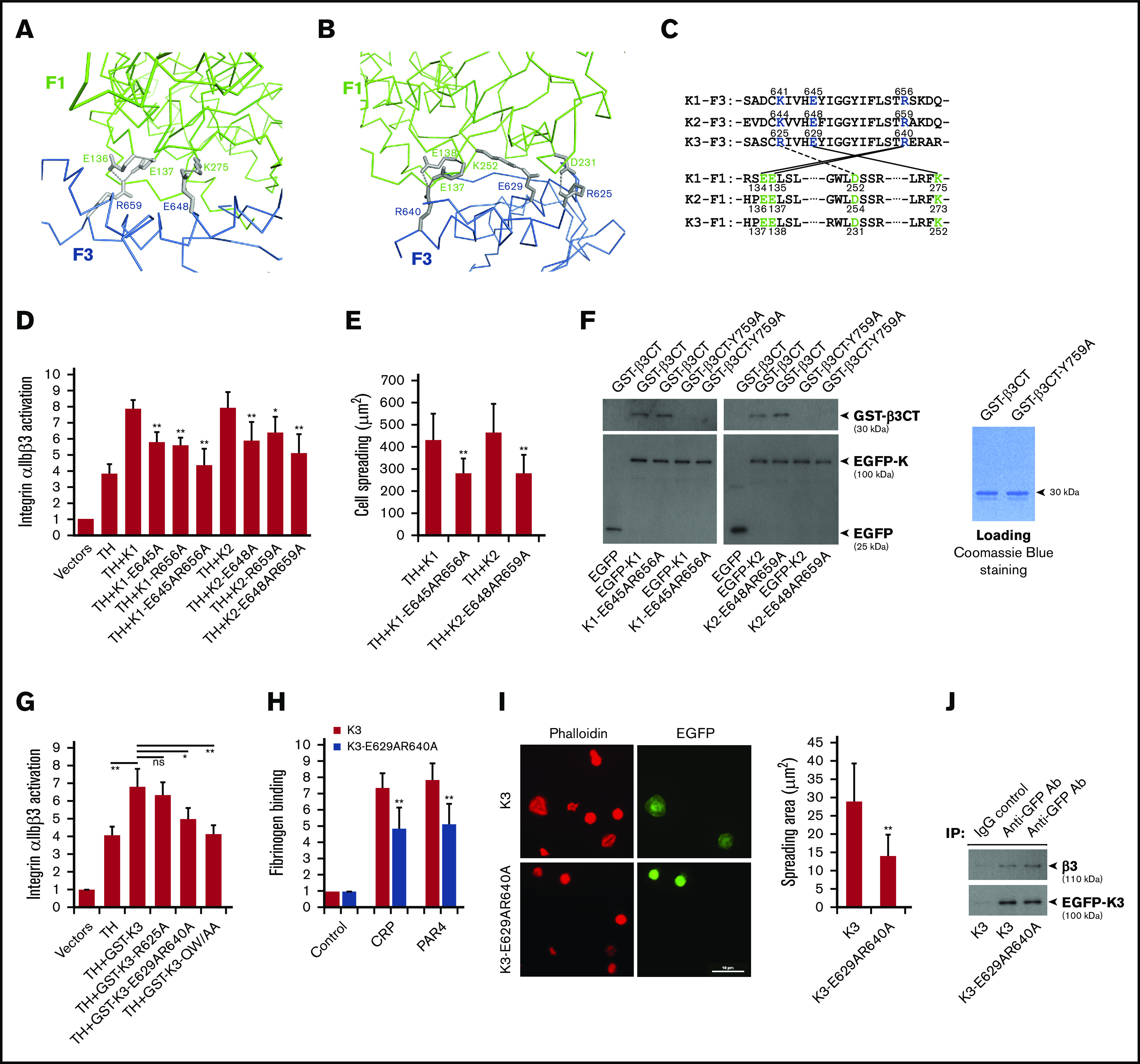
The compact structure of FERM domain in kindlins is required for supporting integrin αIIbβ3 activation. The salt bridges formed at the F1/F3 interface in kindlin-2 (A) and kindlin-3 (B) are shown, in which the side-chain of involved residues are shown in gray and the salt bridges are indicated with dotted lines. (C) The conserved residues mediating the F1/F3 interface in kindlins are positioned in both F1 and F3 subdomains. (D) Integrin αIIbβ3 activation induced by coexpression of the talin head domain (TH) and kindlins, including kindlin-1 (K1) and kindlin-2 (K2) and their mutants, was evaluated by using the PAC-1 binding assay in transfected CHO-αIIbβ3 cells. (E) Integrin αIIbβ3–mediated CHO cell spreading on immobilized fibrinogen was quantified. (F) EGFP-kindlin-1 (K1) and EGFP-kindlin-2 (K2) and their mutants expressed in CHO cells were enriched on protein A/G beads precoated with an anti-EGFP antibody. The beads were then used to incubate with purified GST-β3CT protein and the Y759A mutant, and after extensive washing, interaction between kindlin and GST-β3CT protein was evaluated by using SDS-PAGE and western blotting. (G) The effect of GST-fused EGFP-kindlin-3 (GST-K3) and the indicated mutants on integrin αIIbβ3 activation in CHO-αIIbβ3 cells was evaluated by using the PAC-1 binding assay. (H) Washed platelets from bone marrow–transplanted mice were stimulated with either collagen-related peptide (CRP; 5 µg/mL) or PAR4 agonist peptide (PAR4; 150 µM) and soluble fibrinogen binding to EGFP-positive platelets expressing either EGFP–kindlin-3 (K3) or the designed mutant (K3-E629AR640A) was quantified by fluorescence-activated cell sorting. (I) Washed platelets from bone marrow–transplanted mice were allowed to spread on immobilized fibrinogen in the present to PAR4 agonist peptide (150 µM) for 60 minutes followed by phalloidin staining. Spreading of EGFP-positive platelets expressing either EGFP-kindlin-3 (K3) or the designed mutant (K3-E629AR640A) was calculated. (J) Lysates of platelets expressing EGFP–kindlin-3 (K3) and the designed mutant (K3-E629AR640A) were used to incubate with protein A/G beads precoated with an anti-EGFP antibody. The coprecipitated EGFP–kindlin-3 and integrin αIIbβ3 were then evaluated by using western blotting. Results present the mean ± standard deviation of 3 experiments. *P < .05; **P < .01. IgG, immunoglobulin G.
