Abstract
STUDY QUESTION
To evaluate the implementation of the coding systems in medically assisted reproduction (MAR) centres in the European Union (EU).
SUMMARY ANSWER
Our data show that a significant number of MAR centres use the Single European Code (SEC), but it also shows certain limitations to the coding.
WHAT IS KNOWN ALREADY
Traceability and identification of tissue and cells used for clinical application are extremely important as it is one of the key aspects of quality and safety both for the donors and the recipients. Patients as well as tissues and cells move across the European continent and far beyond, hence a uniform coding system was very much needed. The coding of tissues and cells from human origin was already embedded in the EU directives 2004/23/EC. The use of the Single European Code (SEC) on tissues and cells was enforced in 2017 for tissues and cells distributed within the EU or exported from the EU. The SEC ensures standardization within the EU, allowing the integration of the two existing codes (ISBT-128 and Eurocode) within the SEC structure. Likewise, in the MAR field, the SEC was launched in order to ensure the traceability of reproductive tissues and cells. Gametes and embryos from partner donation as well as reproductive cells and tissues of allogeneic donation were excluded from the SEC as long as they remain in the centre of origin.
STUDY DESIGN, SIZE, DURATION
A cross-sectional survey aimed to gain insight into the use of SEC by MAR centres was conducted between 5 November and 15 December 2018.
PARTICIPANTS/MATERIALS, SETTING, METHODS
The online survey was distributed among the ESHRE members.
MAIN RESULTS AND THE ROLE OF CHANCE
The survey results highlight the strengths and weaknesses in the practical use of the SEC. The data from the survey showed that the SEC code is something that is known in the MAR field. Our data showed that over half of the respondents were using the SEC in their centre. On the other hand, there is also criticism about the use of SEC in MAR, especially that the added value for traceability and identification in ART is found to be rather limited.
LIMITATIONS, REASONS FOR CAUTION
The survey response rate was rather low (4.84%). The view of the use of SEC discussed in this paper still provides insight into the use of the SEC in several MAR centres.
WIDER IMPLICATIONS OF THE FINDINGS
The survey highlights some knowledge gaps concerning coding. This information can be used to develop tools to increase knowledge of the SEC.
STUDY FUNDING/COMPETING INTEREST(S)
There was no external funding for this study. The authors declare that they have no conflict of interest.
TRIAL REGISTRATION NUMBER
N/A.
Keywords: medically assisted reproduction, coding, Single European Code, traceability, laboratory
WHAT DOES THIS MEAN FOR PATIENTS?
The paper describes how coding of samples is applied in Europe, to make sure that there is traceability when reproductive cells for non-partner donations are transported between centres. There are several recommendations and legislation on how this could be done (several codes), and we asked centres using medically assisted reproduction which coding system they use and which problems they encounter using the codes.
The collected information could help to further improve and standardize labelling of samples in the IVF laboratory that are used in non-partner donation. It is not directly relevant for patients, however, indirectly it is of interest as patients who are receiving donated reproductive material that has been transported can be reassured that the traceability chain is complete, from centre of origin to the centre where they receive the treatment.
Introduction
An effective traceability system represents a crucial aspect in medically assisted reproduction (MAR) treatments. In fact, donor selection, collection from the donor, processing, storage and transport of tissues and cells involve many complex steps that impact on the quality and safety of tissues and cells used for human application. For this reason, all steps and procedures to which tissues or cells are subjected need to be identified. Their location and employed equipment and materials need to be recorded before reaching the recipient. The chain of traceability in MAR includes not only the link between donors and recipients but also the follow-up of pregnancies and data on children’s health. Human errors, equipment failures and use of inadequate written procedures can increase safety and health risks not only to recipients but also to offspring. When incidents happen, the ability to track and trace back every step in the process chain helps to find the root cause. In turn, corrective measures can be set up and possible risks in other processes detected and prevented.
The system of traceability is based on a coding system that preferably consists of a globally unique identification code for all donated biological products. In general, gametes and embryos are currently labelled and coded, hence identified, by local or national traceability systems. Although these systems work perfectly well inside a MAR establishment, many people travel abroad to access fertility treatments and this leads to an increase in the cross-border movement not only of patients, but also of tissues and cells (Shenfield et al., 2011). The need for a universal code aimed at connecting the internal traceability chains of two or more MAR centres is essential. A common traceability language, that is understood and readable all over Europe, should facilitate tracing the tissues and cells from donors to recipients and vice versa.
In the European Union (EU), there are three types of coding systems that can be used: (i) ISBT-128, (ii) Eurocode and (iii) Single European Code (SEC) for tissues and cells.
The International Council for Commonality in Blood Banking Automation, an international non-profit non-governmental organization in official relations with the World Health Organization, manages the ISBT-128, which is the most widely used information standard for the coding and labelling of medical products of human origin. It is used in 77 countries across six continents and is endorsed by 21 scientific and professional organizations (Cabana, 2018; Rice, 2018). Recently, a new terminology was developed for the description of MAR products and it is compatible with the ISBT-128 coding system (Ashford et al., 2019).
Eurocode International Blood Labelling Systems e.V. (Eurocode IBLS) is a non-profit organization under German law founded in 1998. Eurocode IBLS manages the coding Eurocode that is established today as a coding system for products of human origin mainly in Germany. It incorporates current International Organization for Standardization (ISO) standards, providing worldwide unique identifiers for labelling blood products, cells and tissues (Knels et al., 2017).
In 2015, the European Commission Directive (EU) 2015/565 amended Directive 2006/86/EC regarding certain technical requirements for the coding of human tissues and cells (European Commission, 2006) , (European Commission, 2015) . The directive introduced the obligation for tissue establishments to use a SEC on tissues and cells distributed for clinical application in the EU or exported from the EU. The SEC ensures the standardization within the EU, allowing the use of ISBT-128 or Eurocode integrated within the SEC coding structure (Caramia et al., 2017).
The SEC should be applied to all tissues and cells used for human application but with some exceptions including cases of partner donation of reproductive cells or when donated tissues and cells remain in the same centre. Other exceptions are when reproductive cells are imported from non-EU countries into the EU in case of an emergency authorized directly by the Competent Authorities, or when tissues and cells that are imported from non-EU countries into the EU remain within the same centre from importation to application, providing that the centre is a tissue establishment that is authorized, designed, accredited or licensed to carry out importing activities.
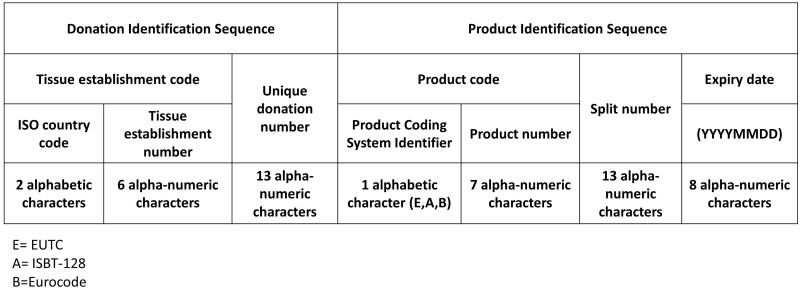
The SEC is a unique identifier consisting of two elements: a donation identification sequence and a product identification sequence (Fig. 1). The donation identification sequence indicates the origin of tissues or cells, mainly because every donor could potentially donate tissues and cells on several occasions (e.g. gamete donation). This sequence is made up of the tissue establishment code, which is composed of the ISO country code in combination with the tissue establishment number allocated in the EU Tissue Establishment Compendium (2020). This represents a register of all tissue establishments that are authorized and licensed by Competent Authorities of the EU member states (accessible through https://webgate.ec.europa.eu/eucoding/reports/te/index.xhtml). Each tissue establishment should assign a unique number for the donation, which is 13 characters in length and based on the donation identification system currently in use in their country. In fact, the donation number may be locally defined or be aligned with national or international standards. The second part of the SEC is a product identification sequence that classifies the type of tissue or cells. It consists of the assigned product code, a split number (if applicable) and the expiry date of the product. The product code includes an identifier of the coding system used. Three systems are approved for use in the SEC: ISBT-128, Eurocode and the European Union Tissues Code (EUTC). Then, the product coding system identifier is ‘A’ for ISBT-128 or ‘B’ for Eurocode or ‘E’ for EUTC, followed by the appropriate product number corresponding to the tissue/cell type, allowing tissue establishments to use one of three product coding systems (ISBT-128, Eurocode, EUTC). The split number is used to identify each product where the donation identification sequence and product code are the same for multiple products, such as multiple cryopreservation devices of oocytes from the same donor. The expiration date of the product is expressed as an eight-digit number using ISO 8601 in International format (YYYYMMDD). Depending on local legislation, the expiry date can be addressed in the SEC. In the case where there is no expiry date described by local legislation, this date should be set to 00000000.
Figure 1.
Explanation of the structure of the Single European Code. ISO, International Organization for Standardization; EUTC, European Union Tissues Code.
The SEC has to be printed in eye-readable format, it should be printed on the label bonded to the tissue or cell packaging, and it has to be recorded in the product-related accompanying documents. Where there is insufficient space for its inclusion on the label, it must appear in accompanying documentation, which is packed together with the product. An example of the SEC is given in Fig. 2.
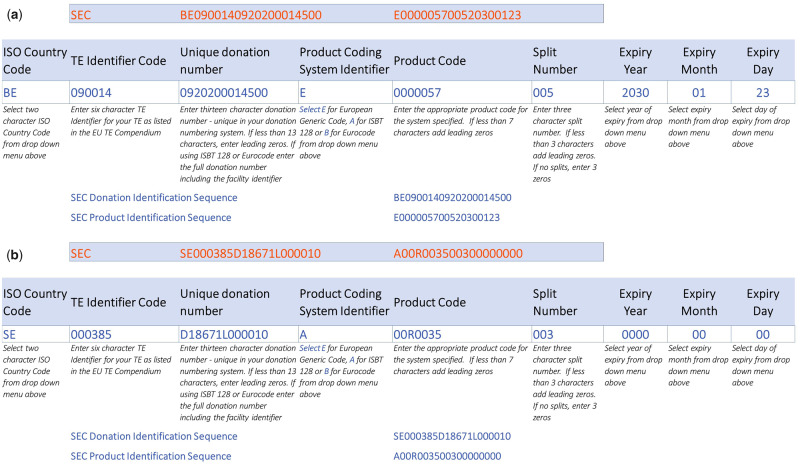
Figure 2.
Example of Single European Code for reproductive cells and tissues on the basis of EUTC and ISBT128. (a) Cryopreservation of a mature oocyte. This oocyte is identified as number 5 in a cohort of 10 oocytes. It has been procured in a Belgian tissue establishment (TE) at Ghent University Hospital that is identified in the Compendium as BE 090014. This TE uses the EUTC to define the product code. The expiry date for oocyte cryopreservation is 10 years and it was cryopreserved by vitrification on 23 January 2020. Additionally, there is a specific cycle number that relates the oocyte vitrification to the stimulation of the patient: 09202000145, which is considered the unique donation code in the SEC. (b) Cryopreservation of a sperm sample (this sample is identified as number 3 in a cohort of 5 from the same ejaculate) in a Swedish TE at Reproductive Medicine, Gothenburg University Hospital. In the Compendium, the TE number is SE00385. Gametes have no expiry date in Sweden, so the date is put as 00000000. The unique donation code is made up by a D (as in ‘Donation’) followed by the specific treatment number, thereafter an L (as in the Swedish word for sequence) followed by an automatically generated sequence number from this clinic: here this was sample number 10 from this clinic. The ISBT-128 code for sperm is then 00R0035.
Application of the SEC by EU tissue establishments has been mandatory since 29 April 2017. However, many establishments have not implemented the coding systems yet. This cross-sectional study was conducted to evaluate the implementation of the coding systems in the EU in MAR centres.
Materials and methods
A cross-sectional study was conducted using an online survey, which focused on the use of coding systems in MAR. The questionnaire was set up by ESHRE and distributed amongst its members.
The survey was set up in SurveyMonkey and included 18 questions organized in two sections: the first focused on the participants’ background and the second on the use of the coding system in MAR.
Participants were asked about denominations and setting of the MAR centre (private or public), country and city of work, and professional career (laboratory director, medical doctor, laboratory technician, embryologist, quality manager, nurse or other).
The survey was open between 5 November and 15 December 2018. Multiple recruitment strategies were used: the study was advertised via two emails, a first specific e-mail to European ESHRE members (n = 3752) and a second email to all ESHRE members using a filter for the European continent (n = 5124) and was accessible via the ESHRE social media.
Results
The survey was sent to 5124 ESHRE members from 114 different countries. A total of 248 people filled out the survey (participation rate 4.84%), representing 38 countries. Most of the participants (96.37%) were from Europe.
The majority of the participants worked in private MAR centres (n = 135/229, 58.95%) while the remainder worked in public centres (n = 94/229, 41.05%). The participants of the survey had different professional roles, with the majority being laboratory directors (n = 95/231, 41.13%) and embryologists (n = 75/231, 32.47%), followed by medical doctors (n = 40/231, 17.32%). Lab technicians, nurses, quality managers and other professional roles accounted for the remaining 9.08% (n = 21/231).
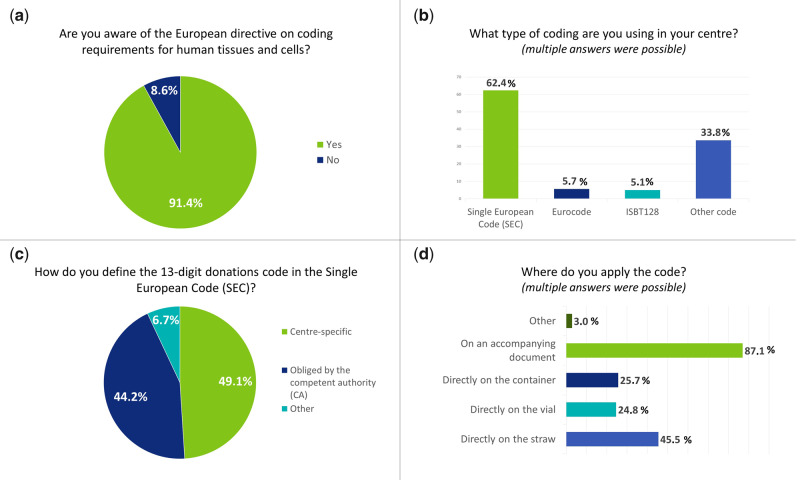
The majority of responders was aware of the European Directive on coding requirements for human tissues and cells (n = 192/210, 91.43%) (Fig. 3a) and used a coding system for traceability in their MAR centres (n = 166/210, 79.05%). Overall, the SEC was used in most centres (n = 98/157, 62.42%) while the use of the Eurocode (n = 9/157, 5.73%) and ISBT-128 (n = 8/157, 5.10%) represented a very small minority (Fig. 3b). The category ‘other code’ was answered in 33.76% (n = 53/157) of the cases. The ‘other code’ category was further explained by 41 participants; 2 replied that they apply a national system, 7 gave no details on the current coding system and 32 reported using an internal code for identifying gametes and embryos. When details of these internal codes were described, eight participants reported including the name of the patients (or other identifier codes for the patients) and/or date of birth. Seven participants reported using SEC in addition to the internal coding for specific samples (donation, samples leaving the centre), and three participants reported being in the process of changing to SEC.
Figure 3.
Detailed answers to four questions of the survey: a) Are you aware of the European directive on coding requirements for human tissues and cells?; b) What type of coding are you using in your centre?; c) How do you define the 13-digit donations code in the SEC; d) Where do you apply the code.
For participants using the SEC code in their practice, a subset of questions on the application of the SEC was posed. The SEC code is composed of a 13-digit donation code that is not specified further in the EU Tissue and Cells Directives (EUTCD). Approximately half of the respondents (n = 30/61, 49.18%) (Fig. 3c), used centre-specific information to fill this 13-digit donation code. Others could not choose how to use this donation code as rules were set up by their Competent Authorities (n = 27/61, 44.26%).
For centres that have implemented the SEC in their activities, in half of the cases the code was used for anonymously donated oocytes (n = 45/99, 45.45%) and sperm (n = 50/99, 50.51%). One-third answered that gametes and embryos for partner donation were also coded using SEC, and 16.16% (n = 16/99) of the participants used the SEC for all types of reproductive cells and tissues. The SEC code was mostly applied when tissues and cells were transported to other centres (n = 66/101, 65.35%) or upon cryopreservation (n = 52/101, 51.49%). Other reasons (n = 14/101, 13.86%) for applying the SEC code were at the start of the MAR treatment cycle, when tissues and cells were collected or when samples were received from other centres. The SEC was mostly applied to an accompanying document (n = 88/101, 87.13%), directly on the straw (n = 46/101, 45.54%) or the vial (n = 25/101, 24.75%) or the container (n = 26/101, 25.74%) (Fig. 3d).
In answering about the difficulties encountered during the implementing of the code in the laboratory procedures, the majority of responders declared that the SEC was too long and difficult to put on devices for cryopreservation. Constructing the code was time-consuming and given the code length, the readability was found to be a major weakness.
Although participants reported some difficulties, 67.65% of the respondents (n = 69/102) found the SEC useful for increasing safety and traceability of cells and tissues during handling and transport between different clinics. The SEC helped with the sample anonymity as writing names on the forms accompanying samples can be avoided. Those in favour of the code found the uniformity across the EU a positive characteristic and probably a first step towards an EU register for donation.
On the contrary, 11.76% of respondents (n = 12/102) found the SEC not useful and found its added value questionable. It would not aid in the identification and traceability as few centres would really check it upon receiving samples. Other identifiers, such as the name of the patient and date of birth or a donor number, were used for tracking the samples. According to 20.59% of participants (n = 21/102), the code could be useful in specific cases, for instance for anonymous donated samples.
The EU coding platform was used by 35.45% (n = 39/110) of respondents, mostly to check the license of tissue establishments where gametes and embryos would be transported to or were originating from.
The major reason for not having implemented one of the coding systems as described in the EUTCD was that most clinics already had an internal coding system for traceability. Some were waiting for their Competent Authority to give guidance on the implementation of a coding system or declared that national legislation did not contain a specific requirement to implement a universal coding system. Others remarked that they would only use it when samples needed to be transported, and this situation had not occurred yet.
Discussion
Although the use of a coding system for traceability of reproductive tissues and cells has been mandatory in the EU since April 2017, not all centres have implemented the EU coding system. There are some exceptions for the use of a coding system in MAR: when tissues and cells are distributed for an immediate clinical application, when donated tissues and cells remain within the same centre, or in the case of reproductive cells from partner donation: the latter is probably the main reason for centres not to implement a universal code, but rather to use an in-house way of identifying gametes and embryos.
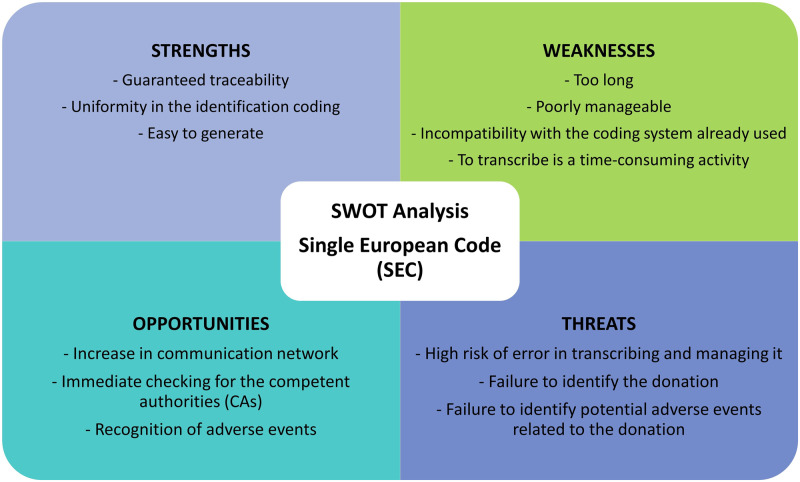
Data from this survey showed that the majority of responders found the use of a code useful. Nevertheless, this survey highlighted many points of criticism. In order to shed light on the different aspects of the SEC management, an SWOT (Strengths, Weaknesses, Opportunities and Threats) analysis was performed (Fig. 4).
Figure 4.
SWOT analysis of the SEC. SWOT, Strengths, Weaknesses, Opportunities and Threats.
The strength of the SEC is its uniform traceability language that can be understood and read throughout multiple countries. Moreover, the SEC seems to be easy enough to be generated by a simple Excel sheet or it can be imbedded into specific software systems. Therefore, the introduction of the SEC in MAR centres should be easy. The SEC increases the communication network for tracking reproductive tissues and cells. Competent Authorities can immediately check and trace the donors and donations. A unique donor identifier facilitates the traceability when serious adverse events or reactions occur. Hence, the SEC goes hand in hand with biovigilance.
Currently, the SEC also entails some weaknesses. It is composed of many digits and a small font size is needed to fit the code on certain labels. To guarantee the traceability, it should be directly administered to packaged tissues or cells. Because of the small devices used for cryopreserved reproductive cells and tissues, the code on such a label is difficult to read, and therefore the true value of using the SEC as an identifier is largely lost. This major weakness may lead to serious adverse consequences. There is a risk of errors during transcribing or deciphering such a long code on the containers of reproductive tissues and cells. In addition, when the code is used on the documentation and a different identifier is used on the straws or vials, this match has to be consistent throughout the traceability chain. Two codes to check and control entail a risk for errors, in comparison to using one code.
Although this survey highlighted critical positive aspects regarding use of the SEC, some limitations should be considered. The survey response rate was rather low (4.84%) and thus the view of the use of SEC discussed in this paper is incomplete. However, the response rate is in line with similar surveys distributed to the full ESHRE membership rather than a specific subgroup (Gameiro et al., 2019). The specific knowledge about SEC in MAR, and especially the lack of use in the daily clinical practice in MAR, could have led to colleagues not replying to the questionnaire. Despite these limitations, we believe that this survey provides insight into the use of the SEC in several MAR centres. This paper can raise awareness of SEC and provide information to centres on the use of SEC.
In conclusion, the role of the SEC is to contribute to the safety of tissue and cells transplant recipients through a system of comprehensive and transparent traceability. Although MAR professionals find the principle of a universal code for traceability very useful, the use of a SEC, ISBT-128 or Eurocode is not yet implemented in all MAR centres. Data from our survey showed that there is a lot of criticism about the use of SEC in the field of MAR, suggesting that tools to increase knowledge of the code are needed. Some centres use the code because it is obligatory, but other identifiers are used for true traceability in the centres.
Acknowledgements
We kindly acknowledge Dr K. Lundin for providing the example on the ISBT-128 SEC.
Authors’ roles
K.T and N.V. designed the study and online survey. A.A and K.T. wrote the manuscript. All authors were involved in data analysis, writing and final approval of the manuscript.
Funding
There was no funding for this work.
Conflict of interest
None declared.
Footnotes
ESHRE pages content is not externally peer reviewed. The manuscript has been approved by the Executive Committee of ESHRE.
References
- Ashford P, Rydman K, Sparks A, Tilleman K, Freire M.. Standard terminology for reproductive tissue and cell products for use in ART. Hum Reprod Open 2019;2019:hoz005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cabana E. (ed). ISBT 128 Standard: Technical Specification. Redlands, CA: ICCBBA, 2018. ISBN 978-1-933243-78-8. www.iccbba.org (14 April 2020, date last accessed). [Google Scholar]
- Caramia V, Ghirardini A, Di Ciaccio P, Vespasiano F, Mareri M, Nanni Costa A.. From the EU legislation to the application of the Single European Code: support to the implementation. Transfus Med Hemother 2017;44:391–394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- EU Tissue Establishment Compendium. https://webgate.ec.europa.eu/eucoding/reports/eugcproduct/index.xhtml (14 April 2020, date. last accessed).
- European Commission. Commission Directive 2006/86/EC of 24 October 2006 implementing Directive 2004/23/EC of the European Parliament and of the Council as regards traceability requirements, notification of serious adverse reactions and events and certain technical requirements for the coding, processing, preservation, storage and distribution of human tissues and cells. Off J Eur Union2006:L294:32–50. http://eur-lex.europa.eu/LexUriServ/ LexUriServ.do?uri=OJ:L:2006:294:0032:0050:EN:PDF (14 April 2020, date last accessed).
- European Commission. Commission Directive (EU) 2015/565 of 8 April 2015 amending Directive 2006/86/EC as regards certain technical requirements for the coding of human tissues and cells. Off J Eur Union2015:L93:43–55. http://eur-lex. europa.eu/legal-content/EN/TXT/PDF/?uri=CELEX:32 015L0565&from=FR (14 April 2020, date last accessed).
- Gameiro S, Sousa-Leite M, Vermeulen N.. Dissemination, implementation and impact of the ESHRE evidence-based guidelines. Hum Reprod Open 2019;2019:hoz011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knels R, Stüpmann K, Pruß A, Klerke J, Kardoeus J, Hiller J.. Coding of tissue and cell preparations using eurocode. Transfus Med Hemother 2017;44:401–405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rice B. (ed). ISBT 128 Standard: Standard Terminology for Medical Products of Human Origin. Redlands, CA: ICCBBA, 2018. [Google Scholar]
- Shenfield F, Pennings G, De Mouzon J, Ferraretti AP, Goossens V; ESHRE Task Force ‘Cross Border Reproductive Care’ (CBRC). ESHRE’s good practice guide for cross-border reproductive care for centers and practitioners. Hum Reprod 2011;26:1625–1627. [DOI] [PubMed] [Google Scholar]