Figure 2.
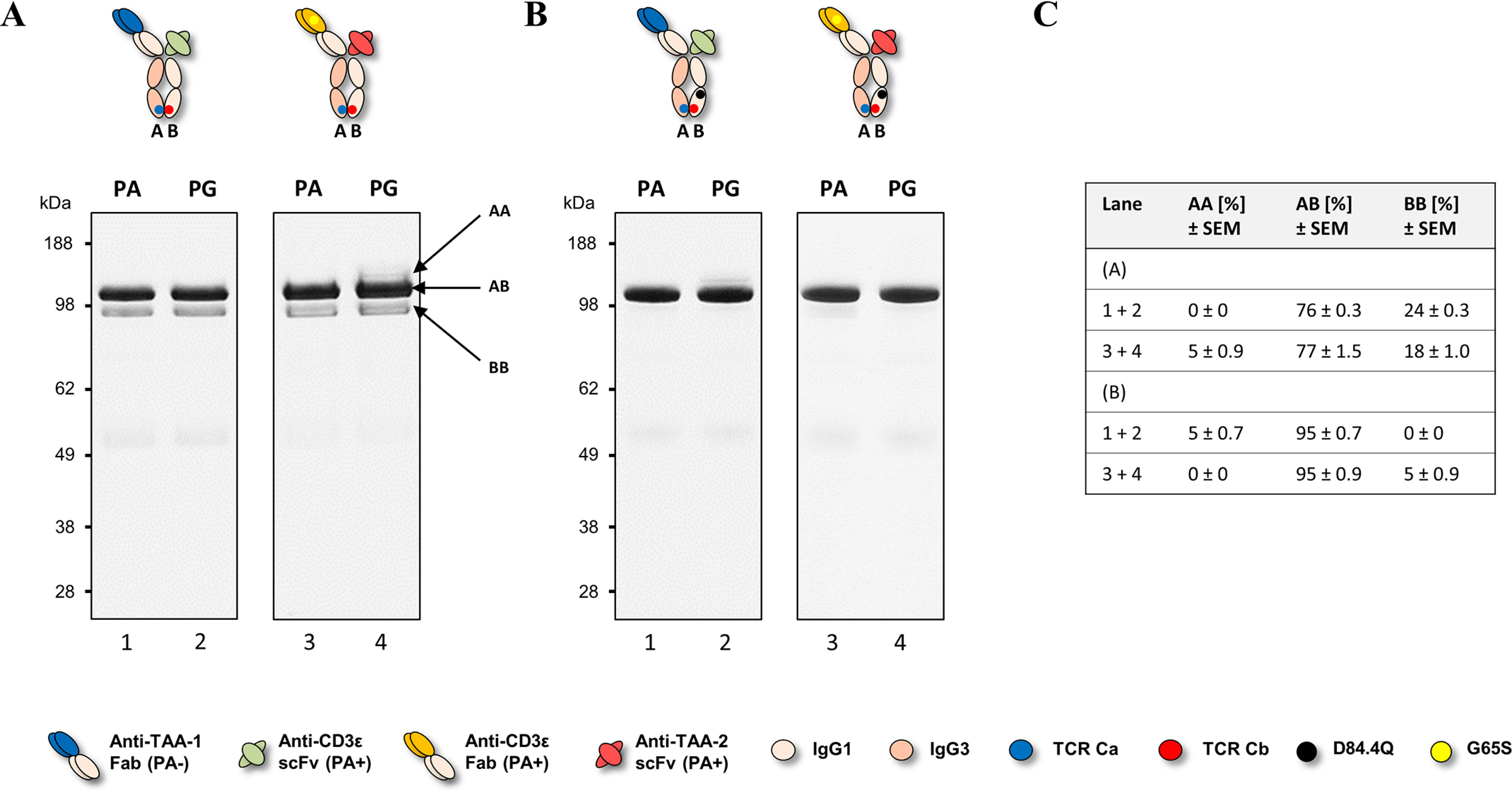
Non-reduced SDS-PAGE analysis of hetero- and homodimer content of two different BEAT constructs. bsAbs were transiently expressed in HEK293-EBNA, purified by PA or PG chromatography, and analyzed by SDS-PAGE. PA+ indicates the presence of a PA-binding site, and PA− indicates the absence of a PA-binding site. For engineering, the G65S mutation was used to abrogate PA binding in VH3-type variable domains when needed. A, lanes 1 and 2, anti–TAA-1 × anti-CD3ε bsAb. Lanes 3 and 4, anti-CD3ε × anti–TAA-2 bsAb. Bands for homodimers (AA and BB) and heterodimers (AB) are annotated with arrows. B, the same bsAbs as in A but carrying the D84.4Q substitution in the CH3 domain of the BEAT (B) chain. C, summary of heterodimer content. Percentages were derived from the combined analysis of the PA and PG pulldowns.
