Figure 4.
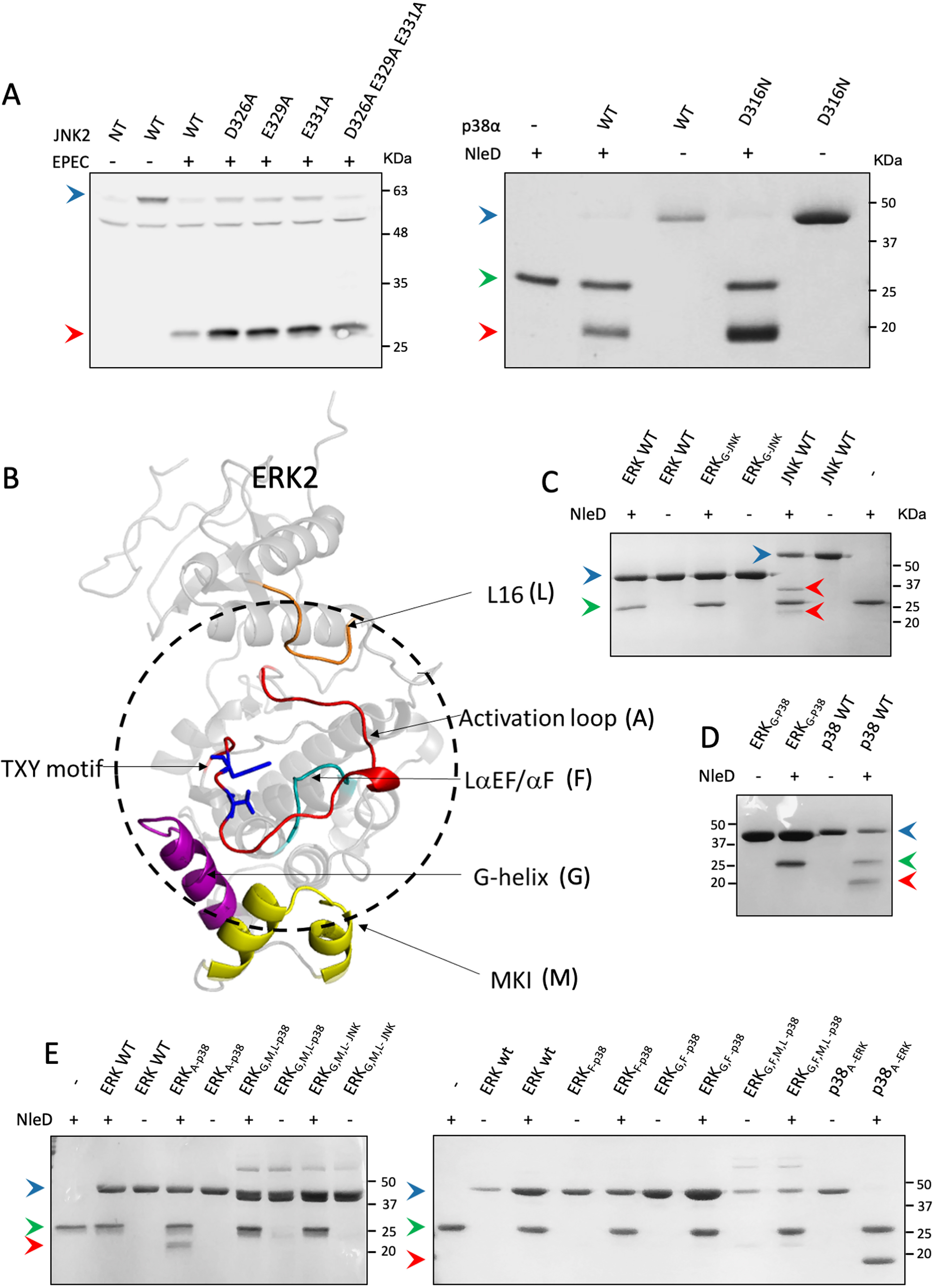
Region replacements between ERK and p38/JNK did not alter cleavage preferences of NleD. A, HEK293T cells were transfected, or not (NT), with plasmids expressing WT JNK2 carrying an HA tag (WT) or JNK2 mutated in critical CD domain residues, as indicated above the lanes (left). The cells were then infected with WT EPEC and subjected to Western blot analysis using an anti-HA antibody. (Right) In addition, WT p38a (WT) or p38 mutated in a critical CD domain residue (D316N) was purified and incubated in vitro with NleDEPEC. Samples were subjected to SDS-PAGE and visualized by Coomassie staining. B, Structure model of ERK2 (PDB entry 4S31). The activation loop is colored red, and threonine and tyrosine of the TXY motif are highlighted in blue. The dashed circle highlights the regions that are in close proximity to the activation loop. Regions that were replaced in the variants include MKI (M), colored yellow, G-helix (G), colored purple, L16 (L), colored orange, and LaEF/aF (F), colored cyan. C–E, Purified MAPK variants were incubated with or without purified NleDEPEC and then resolved by SDS-PAGE, followed by Coomassie staining. The variants used are indicated above the respective lanes and include WT ERK2 (ERKwt), WT JNK2 (JNKwt), WT p38α (p38wt), ERK2 with G-helix of JNK2 or p38α (ERKG-JNK and ERKG-p38, respectively), ERK2 with the p38α activation loop (ERKA-p38), p38α with the ERK2 activation loop (p38A-ERK), ERK2 with the LαEF/αF region of p38α (ERKF-p38), double mutant ERK2 with G-helix and LαEF/αF regions of p38α (ERKG,F-p38), triple mutant ERK2 with G-helix, MKI, and L16 regions of p38α (ERKG,M,l-p38), and quadruple mutant ERK2 with G-helix, LαEF/αF, MKI, and L16 regions of p38α (ERKG,F,M,l-p38). The presence (+) or absence (−) of NleD is indicated. Intact and fragmented MAPKs are indicated by blue and red arrowheads, respectively. A green arrowhead indicates NleD protein.
