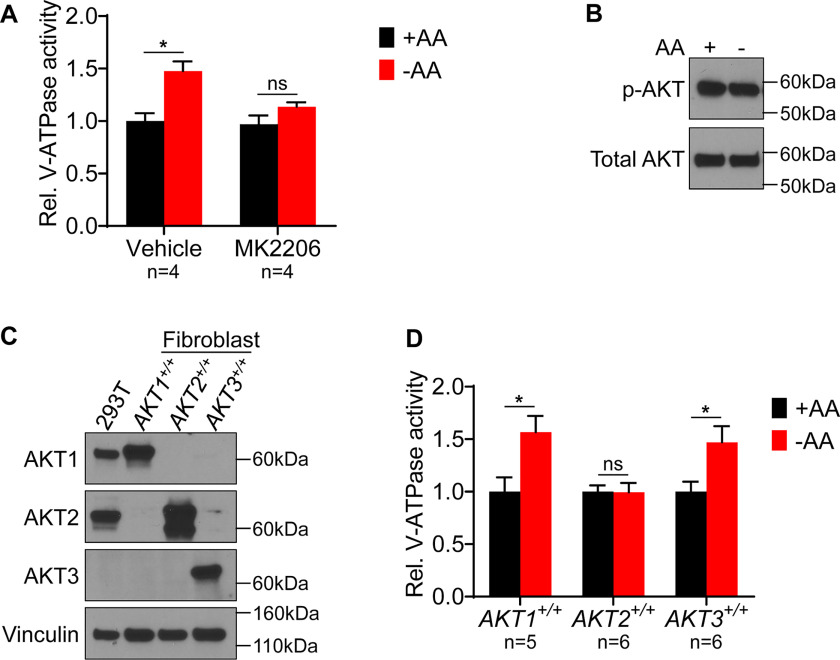
Figure 4.
Effect of AKT inhibition and individual isoform expression on lysosomal V-ATPase activity and response to amino acid starvation. A, The rate of V-ATPase–dependent fluorescence quenching in FITC-dextran-loaded lysosomes was determined for HEK293T cells treated for 1 h with either DMSO (vehicle) or 1 μm MK2206 in the presence (+AA) or absence (−AA) of amino acids. Values are expressed relative to the unstarved, vehicle-treated condition. *, p < 0.05; ns, p ≥ 0.05. Error bars represent S.E. B, HEK293T cells were maintained in amino acids (AA) or starved for 1 h. Lysates were prepared and subjected to Western blotting with the indicated antibodies. The phosphorylated AKT (p-AKT) antibody recognizes AKT1 phospho-S473, AKT2 phospho-S474, and AKT3 phospho-S472. The total AKT antibody recognizes AKT1, AKT2, and AKT3. Representative images are shown (n = 2). C, Western blotting was performed using isoform-specific AKT antibodies on lysates from HEK293T cells and from immortalized mouse lung fibroblasts lacking endogenous AKT and expressing individual human AKT isoforms. Representative images are shown (n = 2). D, Mouse lung fibroblasts lacking endogenous AKT and expressing individual human AKT isoforms were maintained in amino acids (+AA) or starved (−AA) for 1 h, and the rate of V-ATPase–dependent fluorescence quenching in FITC-dextran–loaded lysosomes was determined as described previously. Values are expressed relative to the unstarved condition for each cell line. *, p < 0.05; ns, p ≥ 0.05. Error bars represent S.E.