Fig. 3.
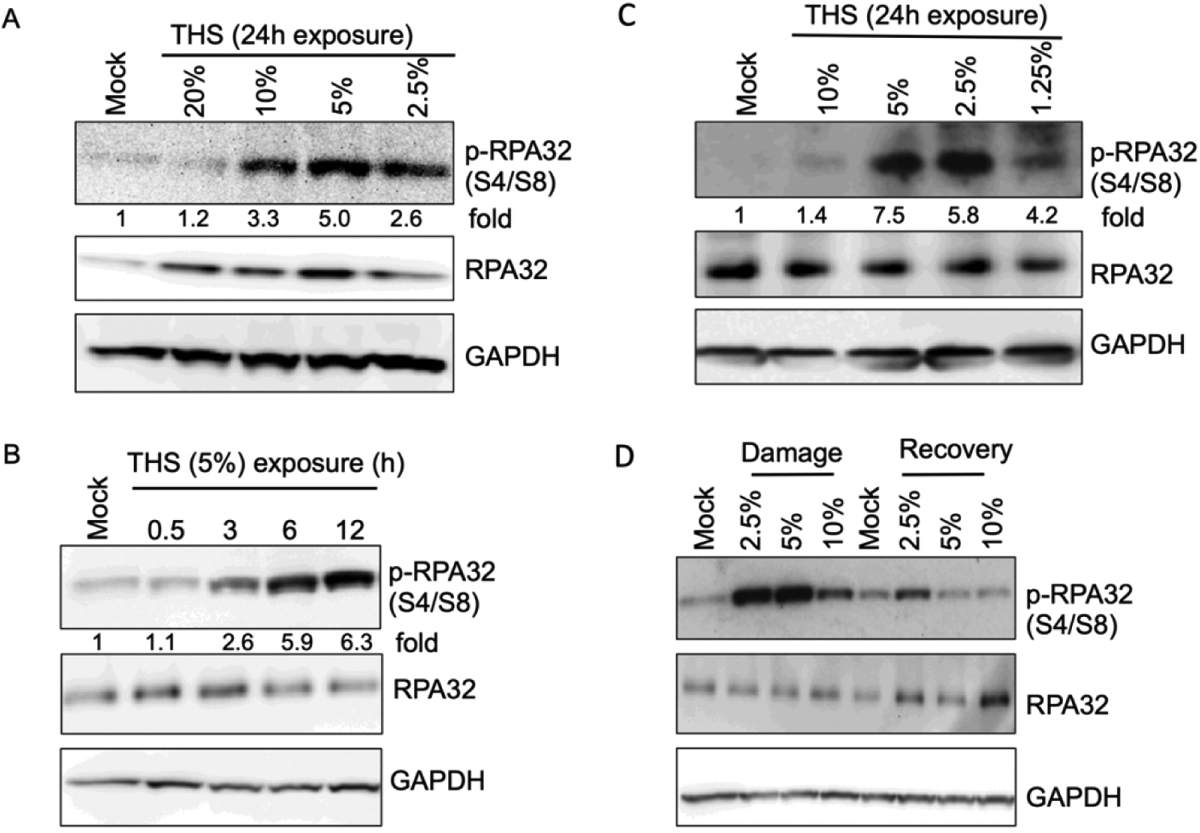
Replication stress following exposure of lung cells to THS as evidenced by RPA phosphorylation. (A) BEAS-2B cells were exposed to various doses of THS as indicated and RPA phosphorylation was monitored by western analysis with phospho-RPA32 (S4/S8) antibody. GAPDH was used as a loading control for normalization of pRPA32 signal at each time point in order to calculate fold increase for each THS dose compared to mock treated control. The same samples were also analyzed with anti-RPA32 antibody to assess the amount of total RPA32. (B). Time course of RPA phosphorylation in BEAS-2B cells exposed to 5% THS for various lengths of time. Cells were collected at the indicated treatment times followed by western analysis with phospho-RPA32 (S4/S8) antibody. Fold increase in RPA phosphorylation was calculated after normalization with GAPDH. (C) hPFs were exposed to the indicated doses of THS and RPA phosphorylation was monitored as in panel A. (D) Recovery of hPFs cells from replication stress following exposure to various doses of THS. Cells were treated with THS for 12h or mock treated, then allowed to recover for 12h by changing to complete medium. Induction of pRPA32 and recovery as indicated by its loss were followed by western analysis using phospho-RPA32 (S4/S8) antibody.
